PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice
- PMID: 21300912
- PMCID: PMC3039848
- DOI: 10.1084/jem.20100466
PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice
Abstract
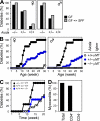
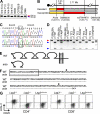
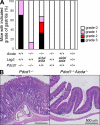
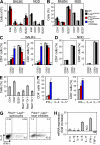
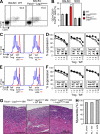
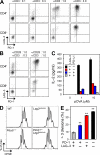
Stimulatory and inhibitory co-receptors play fundamental roles in the regulation of the immune system. We describe a new mouse model of spontaneous autoimmune disease. Activation-induced cytidine deaminase-linked autoimmunity (aida) mice harbor a loss-of-function mutation in the gene encoding lymphocyte activation gene 3 (LAG-3), an inhibitory co-receptor. Although LAG-3 deficiency alone did not induce autoimmunity in nonautoimmune-prone mouse strains, it induced lethal myocarditis in BALB/c mice deficient for the gene encoding the inhibitory co-receptor programmed cell death 1 (PD-1). In addition, LAG-3 deficiency alone accelerated type 1 diabetes mellitus in nonobese diabetic mice. These results demonstrate that LAG-3 acts synergistically with PD-1 and/or other immunoregulatory genes to prevent autoimmunity in mice.
Figures



Similar articles
-
Forced expression of programmed death-1 gene on T cell decreased the incidence of type 1 diabetes.Arch Pharm Res. 2010 Nov;33(11):1825-33. doi: 10.1007/s12272-010-1115-3. Epub 2010 Nov 30. Arch Pharm Res. 2010. PMID: 21116786
-
On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection.Int Immunol. 2010 Jan;22(1):13-23. doi: 10.1093/intimm/dxp107. Epub 2009 Oct 30. Int Immunol. 2010. PMID: 19880580
-
Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival.Transplantation. 2003 Sep 27;76(6):994-9. doi: 10.1097/01.TP.0000085010.39567.FB. Transplantation. 2003. PMID: 14508368
-
New regulatory co-receptors: inducible co-stimulator and PD-1.Curr Opin Immunol. 2002 Dec;14(6):779-82. doi: 10.1016/s0952-7915(02)00398-9. Curr Opin Immunol. 2002. PMID: 12413529 Review.
-
The reverse stop-signal model for CTLA4 function.Nat Rev Immunol. 2008 Feb;8(2):153-60. doi: 10.1038/nri2253. Nat Rev Immunol. 2008. PMID: 18219311 Review.
Cited by
-
Molecular signatures of T-cell inhibition in HIV-1 infection.Retrovirology. 2013 Mar 20;10:31. doi: 10.1186/1742-4690-10-31. Retrovirology. 2013. PMID: 23514593 Free PMC article. Review.
-
Activation-induced cytidine deaminase expression by thymic B cells promotes T-cell tolerance and limits autoimmunity.iScience. 2022 Dec 21;26(1):105852. doi: 10.1016/j.isci.2022.105852. eCollection 2023 Jan 20. iScience. 2022. PMID: 36654860 Free PMC article.
-
Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: Monotherapies and Combined Therapies.Front Oncol. 2022 Jun 16;12:898964. doi: 10.3389/fonc.2022.898964. eCollection 2022. Front Oncol. 2022. PMID: 35785169 Free PMC article. Review.
-
Immune checkpoint inhibitor-associated cardiovascular toxicities: A review.Heliyon. 2024 Feb 9;10(5):e25747. doi: 10.1016/j.heliyon.2024.e25747. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38434280 Free PMC article. Review.
-
Gene expression profiling of the response to interferon beta in Epstein-Barr-transformed and primary B cells of patients with multiple sclerosis.PLoS One. 2014 Jul 15;9(7):e102331. doi: 10.1371/journal.pone.0102331. eCollection 2014. PLoS One. 2014. PMID: 25025430 Free PMC article.
References
-
- Akashi T., Nagafuchi S., Anzai K., Kondo S., Kitamura D., Wakana S., Ono J., Kikuchi M., Niho Y., Watanabe T. 1997. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int. Immunol. 9:1159–1164 10.1093/intimm/9.8.1159 - DOI - PubMed
-
- Bachmann M.F., Köhler G., Ecabert B., Mak T.W., Kopf M. 1999. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol. 163:1128–1131 - PubMed
-
- Baixeras E., Huard B., Miossec C., Jitsukawa S., Martin M., Hercend T., Auffray C., Triebel F., Piatier-Tonneau D. 1992. Characterization of the lymphocyte activation gene 3–encoded protein. A new ligand for human leukocyte antigen class II antigens. J. Exp. Med. 176:327–337 10.1084/jem.176.2.327 - DOI - PMC - PubMed
-
- Brunkow M.E., Jeffery E.W., Hjerrild K.A., Paeper B., Clark L.B., Yasayko S.A., Wilkinson J.E., Galas D., Ziegler S.F., Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73 10.1038/83784 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

