Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907
- PMID: 21300923
- PMCID: PMC3068052
- DOI: 10.1200/JCO.2010.29.9859
Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907
Abstract
Purpose: A phase III trial (Cancer and Leukemia Group B CALGB-49907) was conducted to test whether older patients with early-stage breast cancer would have equivalent relapse-free and overall survival with capecitabine compared with standard chemotherapy. The quality of life (QoL) substudy tested whether capecitabine treatment would be associated with a better QoL than standard chemotherapy.
Patients and methods: QoL was assessed in 350 patients randomly assigned to either standard chemotherapy (cyclophosphamide, methotrexate, and fluorouracil [CMF] or doxorubicin and cyclophosphamide [AC]; n = 182) or capecitabine (n = 168). Patients were interviewed by telephone before treatment (baseline), midtreatment, within 1 month post-treatment, and at 12, 18, and 24 months postbaseline by using questionnaires from the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30), a breast systemic adverse effects scale (EORTC BR23), and the Hospital Anxiety and Depression Scale (HADS).
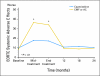
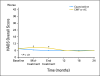
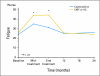
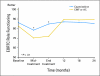
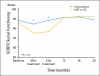
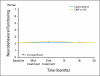
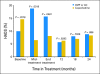
Results: Compared with patients who were treated with standard chemotherapy, patients who were treated with capecitabine had significantly better QoL, role function, and social function, fewer systemic adverse effects, less psychological distress, and less fatigue during and at the completion of treatment (P ≤ .005). Capecitabine treatment was associated with less nausea, vomiting, and constipation and with better appetite than standard treatment (P ≤ .004), but worse hand-foot syndrome and diarrhea (P < .005). These differences all resolved by 12 months.
Conclusion: Standard chemotherapy was superior to capecitabine in improving relapse-free and overall survival for older women with early-stage breast cancer. Although capecitabine was associated with better QoL during treatment, QoL was similar for both groups at 1 year. The brief period of poorer QoL with standard treatment is a modest price to pay for a chance at improved survival.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. - PubMed
-
- Oshaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate, and 5-fluououracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–1254. - PubMed
-
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. - PubMed
-
- Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J Clin Oncol. 1996;14:2756–2768. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- CA32291/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- U10 CA085850/CA/NCI NIH HHS/United States
- P30 AG028716/AG/NIA NIH HHS/United States
- U10CA85850/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA047577/CA/NCI NIH HHS/United States
- U10 CA032291/CA/NCI NIH HHS/United States
- CA77406/CA/NCI NIH HHS/United States
- P30-AG-028716/AG/NIA NIH HHS/United States
- CA47577/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- CA77651/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

