Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial
- PMID: 21300930
- PMCID: PMC3083866
- DOI: 10.1200/JCO.2010.29.7689
Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial
Abstract
Purpose: Premenopausal women with breast cancer receiving adjuvant chemotherapy are at risk for amenorrhea. The National Surgical Adjuvant Breast and Bowel Project B-30 trial included menstrual history (MH) and quality-of-life (QOL) studies to compare treatments on these outcomes.
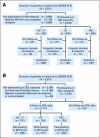
Patients and methods: Patients were randomly assigned to sequential doxorubicin (A) and cyclophosphamide (C) followed by docetaxel (T; AC→T), concurrent TAC, or AT, which varied in duration (24, 12, 12 weeks, respectively), and use of C. Endocrine therapy was prescribed for women with hormone receptor-positive tumors. MH and QOL were assessed with standardized questionnaires at baseline; cycle 4, day 1; and every 6 months through 24 months. Prespecified analyses examined rates of amenorrhea by treatment arm, the relationship between amenorrhea and QOL, and QOL by treatment arm.
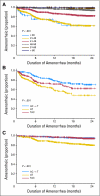
Results: Amenorrhea 12 months after random assignment was significantly different between treatment groups: 69.8% for AC→T, 57.7% for TAC, and 37.9% for AT (P < .001). The AT group without tamoxifen had the lowest rate of amenorrhea. QOL was poorer for patients receiving AC→T at 6 months but similar to others by 12 months. Post-treatment symptoms were increased above baseline for all treatments. Multivariable repeated measures modeling demonstrated that treatment arm, time point, age, and tamoxifen use were significantly associated with symptom severity (all P values < .002).
Conclusion: Amenorrhea rates differed significantly by treatment arm, with the AT arm having the lowest rate. Patients treated with longer duration therapy (AC→T) had greater symptom severity and poorer QOL at 6 months, but did not differ from shorter duration treatments at 12 months.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Life after adjuvant chemotherapy for breast cancer: the news is mostly good.J Clin Oncol. 2011 Mar 20;29(9):1092-3. doi: 10.1200/JCO.2010.33.5588. Epub 2011 Feb 7. J Clin Oncol. 2011. PMID: 21300925 No abstract available.
References
-
- Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. - PubMed
-
- Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: Understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. - PubMed
-
- Ganz PA. Menopause and breast cancer: Symptoms, late effects, and their management. Semin Oncol. 2001;28:274–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

