Stochastic dynamics of cancer initiation
- PMID: 21301064
- PMCID: PMC3569097
- DOI: 10.1088/1478-3975/8/1/015002
Stochastic dynamics of cancer initiation
Abstract
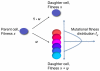
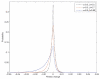
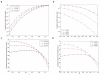
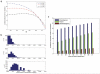
Most human cancer types result from the accumulation of multiple genetic and epigenetic alterations in a single cell. Once the first change (or changes) have arisen, tumorigenesis is initiated and the subsequent emergence of additional alterations drives progression to more aggressive and ultimately invasive phenotypes. Elucidation of the dynamics of cancer initiation is of importance for an understanding of tumor evolution and cancer incidence data. In this paper, we develop a novel mathematical framework to study the processes of cancer initiation. Cells at risk of accumulating oncogenic mutations are organized into small compartments of cells and proliferate according to a stochastic process. During each cell division, an (epi)genetic alteration may arise which leads to a random fitness change, drawn from a probability distribution. Cancer is initiated when a cell gains a fitness sufficiently high to escape from the homeostatic mechanisms of the cell compartment. To investigate cancer initiation during a human lifetime, a 'race' between this fitness process and the aging process of the patient is considered; the latter is modeled as a second stochastic Markov process in an aging dimension. This model allows us to investigate the dynamics of cancer initiation and its dependence on the mutational fitness distribution. Our framework also provides a methodology to assess the effects of different life expectancy distributions on lifetime cancer incidence. We apply this methodology to colorectal tumorigenesis while considering life expectancy data of the US population to inform the dynamics of the aging process. We study how the probability of cancer initiation prior to death, the time until cancer initiation, and the mutational profile of the cancer-initiating cell depends on the shape of the mutational fitness distribution and life expectancy of the population.
Figures

Similar articles
-
An Evolutionary Approach for Identifying Driver Mutations in Colorectal Cancer.PLoS Comput Biol. 2015 Sep 17;11(9):e1004350. doi: 10.1371/journal.pcbi.1004350. eCollection 2015 Sep. PLoS Comput Biol. 2015. PMID: 26379039 Free PMC article.
-
Evolutionary dynamics of tumor progression with random fitness values.Theor Popul Biol. 2010 Aug;78(1):54-66. doi: 10.1016/j.tpb.2010.05.001. Epub 2010 May 19. Theor Popul Biol. 2010. PMID: 20488197 Free PMC article.
-
Mutations, evolution and the central role of a self-defined fitness function in the initiation and progression of cancer.Biochim Biophys Acta Rev Cancer. 2017 Apr;1867(2):162-166. doi: 10.1016/j.bbcan.2017.03.005. Epub 2017 Mar 21. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28341421 Free PMC article. Review.
-
Stochastic Tunneling of Two Mutations in a Population of Cancer Cells.PLoS One. 2013 Jun 26;8(6):e65724. doi: 10.1371/journal.pone.0065724. Print 2013. PLoS One. 2013. PMID: 23840359 Free PMC article.
-
Declining cellular fitness with age promotes cancer initiation by selecting for adaptive oncogenic mutations.Biochim Biophys Acta. 2008 Jan;1785(1):1-11. doi: 10.1016/j.bbcan.2007.09.001. Epub 2007 Oct 12. Biochim Biophys Acta. 2008. PMID: 17980163 Free PMC article. Review.
Cited by
-
Effect of dedifferentiation on time to mutation acquisition in stem cell-driven cancers.PLoS Comput Biol. 2014 Mar 6;10(3):e1003481. doi: 10.1371/journal.pcbi.1003481. eCollection 2014 Mar. PLoS Comput Biol. 2014. PMID: 24603301 Free PMC article.
-
An Evolutionary Approach for Identifying Driver Mutations in Colorectal Cancer.PLoS Comput Biol. 2015 Sep 17;11(9):e1004350. doi: 10.1371/journal.pcbi.1004350. eCollection 2015 Sep. PLoS Comput Biol. 2015. PMID: 26379039 Free PMC article.
-
Eleven grand challenges in single-cell data science.Genome Biol. 2020 Feb 7;21(1):31. doi: 10.1186/s13059-020-1926-6. Genome Biol. 2020. PMID: 32033589 Free PMC article. Review.
-
The implications of small stem cell niche sizes and the distribution of fitness effects of new mutations in aging and tumorigenesis.Evol Appl. 2016 Mar 8;9(4):565-82. doi: 10.1111/eva.12361. eCollection 2016 Apr. Evol Appl. 2016. PMID: 27099622 Free PMC article.
-
Dynamic Emergence of Observed and Hidden Intra-tumor Heterogeneity.iScience. 2019 Nov 22;21:157-167. doi: 10.1016/j.isci.2019.10.018. Epub 2019 Oct 10. iScience. 2019. PMID: 31655256 Free PMC article.
References
-
- Mintz B. Clonal basis of mammalian differentiation. Symp. Soc. Exp. Biol. 1971;25:345–370. - PubMed
-
- Mintz B. Malignancy versus normal differentiation of stem cells as analyzed in genetically mosaic animals. Adv. Pathobiol. 1977;6:153–157. - PubMed
-
- Kovacs L, Potten CS. An estimation of proliferative population size in stomach, jejenum and colon of dba-2 mice. Cell Tissue Kinet. 1973;6:125–134. - PubMed
-
- Bach SP, Renehan AG, Potten CS. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis. 2000;21:469–476. - PubMed
-
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
