Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: current strategies
- PMID: 21301207
- PMCID: PMC3084968
- DOI: 10.4161/cbt.11.7.14948
Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: current strategies
Abstract
Many solid tumors and hematologic malignancies lack expression of the enzyme methylthioadenosine phosphorylase (MTAP), due either to deletion of the MTAP gene or to methylation of the MTAP promoter. In cells that have MTAP, its natural substrate, methylthioadenosine (MTA), generated during polyamine biosynthesis, is cleaved to adenine and 5-methylthioribose-1-phosphate. The latter compound is further metabolized to methionine. Adenine and methionine are further metabolized and hence salvaged. In MTAP-deficient cells, however, MTA is not cleaved and the salvage pathway for adenine and methionine is absent. As a result, MTAP-deficient cells are more sensitive than MTAP-positive cells to inhibitors of de novo purine synthesis and to methionine deprivation. The challenge has been to take advantage of MTAP deficiency, and the changes in metabolism that follow, to design a strategy for targeted treatment. In this review, the frequency of MTAP-deficiency is presented and past and recent strategies to target such deficient cells are discussed, including one in which MTA is administered, followed by very high doses of a toxic purine or pyrimidine analog. In normal host cells, adenine, generated from MTA, blocks conversion of the analog to its toxic nucleotide. In MTAP-deficient tumor cells, conversion proceeds and the tumor cells are selectively killed. Successful mouse studies using this novel strategy were recently reported.
Figures
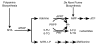
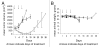
Similar articles
-
Selective killing of tumors deficient in methylthioadenosine phosphorylase: a novel strategy.PLoS One. 2009 May 29;4(5):e5735. doi: 10.1371/journal.pone.0005735. PLoS One. 2009. PMID: 19478948 Free PMC article.
-
Expression of methylthioadenosine phosphorylase cDNA in p16-, MTAP- malignant cells: restoration of methylthioadenosine phosphorylase-dependent salvage pathways and alterations of sensitivity to inhibitors of purine de novo synthesis.Mol Pharmacol. 1997 Nov;52(5):903-11. doi: 10.1124/mol.52.5.903. Mol Pharmacol. 1997. PMID: 9351982
-
Lack of expression of MTAP in uncommon T-cell lymphomas.Clin Lymphoma Myeloma Leuk. 2012 Oct;12(5):306-9. doi: 10.1016/j.clml.2012.07.001. Clin Lymphoma Myeloma Leuk. 2012. PMID: 23040436
-
Methylthioadenosine phosphorylase deficiency in tumors: A compelling therapeutic target.Front Cell Dev Biol. 2023 Apr 5;11:1173356. doi: 10.3389/fcell.2023.1173356. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37091983 Free PMC article. Review.
-
MTAP as an emerging biomarker in thoracic malignancies.Lung Cancer. 2024 Nov;197:107963. doi: 10.1016/j.lungcan.2024.107963. Epub 2024 Sep 29. Lung Cancer. 2024. PMID: 39357262 Review.
Cited by
-
Metabolic Regulation as a Consequence of Anaerobic 5-Methylthioadenosine Recycling in Rhodospirillum rubrum.mBio. 2016 Jul 12;7(4):e00855-16. doi: 10.1128/mBio.00855-16. mBio. 2016. PMID: 27406564 Free PMC article.
-
Diagnostic and Prognostic Significance of Methionine Uptake and Methionine Positron Emission Tomography Imaging in Gliomas.Front Oncol. 2017 Nov 1;7:257. doi: 10.3389/fonc.2017.00257. eCollection 2017. Front Oncol. 2017. PMID: 29164057 Free PMC article. Review.
-
Specific Targeting of MTAP-Deleted Tumors with a Combination of 2'-Fluoroadenine and 5'-Methylthioadenosine.Cancer Res. 2018 Aug 1;78(15):4386-4395. doi: 10.1158/0008-5472.CAN-18-0814. Epub 2018 May 29. Cancer Res. 2018. PMID: 29844120 Free PMC article.
-
The prognostic role of intragenic copy number breakpoints and identification of novel fusion genes in paediatric high grade glioma.Acta Neuropathol Commun. 2014 Feb 18;2:23. doi: 10.1186/2051-5960-2-23. Acta Neuropathol Commun. 2014. PMID: 24548782 Free PMC article.
-
A triple-punch approach: methionine restriction enhances combination inhibitors in brain metastatic triple-negative breast cancer.J Clin Invest. 2025 Jul 1;135(13):e193171. doi: 10.1172/JCI193171. eCollection 2025 Jul 1. J Clin Invest. 2025. PMID: 40590221 Free PMC article.
References
-
- Carson DA, Nobori T, Kajander EO, Carrera CJ, Kubota M, Yamanaka H. Methylthioadenosine (MeSAdo) phosphorylase deficiency in malignancy. Adv Exp Med Biol. 1988;250:179–185. - PubMed
-
- Kindler HL, Burris HA, III, Sandler AB, Oliff IA. A phase II multicenter study of L-alanosine, a potent inhibitor of adenine biosynthesis, in patients with MTAP-deficient cancer. Invest New Drugs. 2009;27:75–81. - PubMed
-
- Chen ZH, Zhang H, Savarese TM. Gene deletion chemoselectivity: codeletion of the genes for p16(INK4), methylthioadenosine phosphorylase and the alpha and beta-interferons in human pancreatic cell carcinoma cell lines and its implications for chemotherapy. Cancer Res. 1996;56:1083–1090. - PubMed
-
- Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108–2113. - PubMed
-
- Hustinx SR, Hruban RH, Leoni LM, Iacobuzio-Donahue C, Cameron JL, Yeo CJ, et al. Homozygous deletion of the MTAP gene in invasive adenocarcinoma of the pancreas and in peri-ampullary cancer: a potential new target for chemotherapy. Cancer Biol Ther. 2005;4:83–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
