Structure-based design of a heptavalent anthrax toxin inhibitor
- PMID: 21302959
- PMCID: PMC3095510
- DOI: 10.1021/bm101396u
Structure-based design of a heptavalent anthrax toxin inhibitor
Abstract
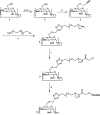
The design of polyvalent molecules, consisting of multiple copies of a biospecific ligand attached to a suitable scaffold, represents a promising approach to inhibit pathogens and oligomeric microbial toxins. Despite the increasing interest in structure-based drug design, few polyvalent inhibitors based on this approach have shown efficacy in vivo. Here we demonstrate the structure-based design of potent biospecific heptavalent inhibitors of anthrax lethal toxin. Specifically, we illustrate the ability to design potent polyvalent ligands by matching the pattern of binding sites on the biological target. We used a combination of experimental studies based on mutagenesis and computational docking studies to identify the binding site for an inhibitory peptide on the heptameric subunit of anthrax toxin. We developed an approach based on copper-catalyzed azide-alkyne cycloaddition (click-chemistry) to facilitate the attachment of seven copies of the inhibitory peptide to a β-cyclodextrin core via a polyethylene glycol linker of an appropriate length. The resulting heptavalent inhibitors neutralized anthrax lethal toxin both in vitro and in vivo and showed appreciable stability in serum. Given the inherent biocompatibility of cyclodextrin and polyethylene glycol, these potent well-defined heptavalent inhibitors show considerable promise as anthrax antitoxins.
Figures





References
-
- Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 2007;76:243–265. - PubMed
-
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JAT. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. - PubMed
-
- Collier RJ, Young JAT. Anthrax Toxin. Annu. Rev. Cell Dev. Biol. 2003;19:45–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

