Noninvasive multimodal evaluation of bioengineered cartilage constructs combining time-resolved fluorescence and ultrasound imaging
- PMID: 21303258
- PMCID: PMC3065732
- DOI: 10.1089/ten.tec.2010.0368
Noninvasive multimodal evaluation of bioengineered cartilage constructs combining time-resolved fluorescence and ultrasound imaging
Abstract
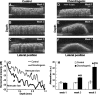
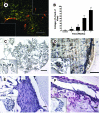
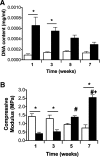
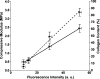
A multimodal diagnostic system that integrates time-resolved fluorescence spectroscopy, fluorescence lifetime imaging microscopy, and ultrasound backscatter microscopy is evaluated here as a potential tool for assessing changes in engineered tissue composition and microstructure nondestructively and noninvasively. The development of techniques capable of monitoring the quality of engineered tissue, determined by extracellular matrix (ECM) content, before implantation would alleviate the need for destructive assays over multiple time points and advance the widespread development and clinical application of engineered tissues. Using a prototype system combining time-resolved fluorescence spectroscopy, FLIM, and UBM, we measured changes in ECM content occurring during chondrogenic differentiation of equine adipose stem cells on 3D biodegradable matrices. The optical and ultrasound results were validated against those acquired via conventional techniques, including collagen II immunohistochemistry, picrosirius red staining, and measurement of construct stiffness. Current results confirm the ability of this multimodal approach to follow the progression of tissue maturation along the chondrogenic lineage by monitoring ECM production (namely, collagen type II) and by detecting resulting changes in mechanical properties of tissue constructs. Although this study was directed toward monitoring chondrogenic tissue maturation, these data demonstrate the feasibility of this approach for multiple applications toward engineering other tissues, including bone and vascular grafts.
Figures


References
-
- Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. - PubMed
-
- Atala A. Engineering organs. Curr Opin Biotechnol. 2009;20:575. - PubMed
-
- Andersson-Engels S. Johansson J. Stenram U. Svanberg K. Svanberg S. Time-resolved laser-induced fluorescence spectroscopy for enhanced demarcation of human atherosclerotic plaques. J Photochem Photobiol B. 1990;4:363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

