Phosphoethanolamine methyltransferases in phosphocholine biosynthesis: functions and potential for antiparasite therapy
- PMID: 21303393
- PMCID: PMC4107886
- DOI: 10.1111/j.1574-6976.2011.00267.x
Phosphoethanolamine methyltransferases in phosphocholine biosynthesis: functions and potential for antiparasite therapy
Abstract
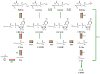
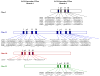
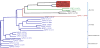
S-adenosyl-L-methionine (SAM)-dependent methyltransferases represent a diverse group of enzymes that catalyze the transfer of a methyl group from a methyl donor SAM to nitrogen, oxygen, sulfur or carbon atoms of a large number of biologically active large and small molecules. These modifications play a major role in the regulation of various biological functions such as gene expression, signaling, nuclear division and metabolism. The three-step SAM-dependent methylation of phosphoethanolamine to form phosphocholine catalyzed by phosphoethanolamine N-methyltransferases (PMTs) has emerged as an important biochemical step in the synthesis of the major phospholipid, phosphatidylcholine, in some eukaryotes. PMTs have been identified in nematodes, plants, African clawed frogs, zebrafish, the Florida lancelet, Proteobacteria and human malaria parasites. Data accumulated thus far suggest an important role for these enzymes in growth and development. This review summarizes published studies on the biochemical and genetic characterization of these enzymes, and discusses their evolution and their suitability as targets for the development of therapies against parasitic infections, as well as in bioengineering for the development of nutritional and stress-resistant plants.
© 2011 Federation of European Microbiological Societies. Published by Blackwell Publishing Ltd. All rights reserved.
Figures