Concomitant influence of helminth infection and landscape on the distribution of Puumala hantavirus in its reservoir, Myodes glareolus
- PMID: 21303497
- PMCID: PMC3040693
- DOI: 10.1186/1471-2180-11-30
Concomitant influence of helminth infection and landscape on the distribution of Puumala hantavirus in its reservoir, Myodes glareolus
Abstract
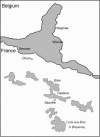
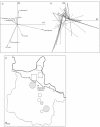
Background: Puumala virus, the agent of nephropathia epidemica (NE), is the most prevalent hantavirus in Europe. The risk for human infection seems to be strongly correlated with the prevalence of Puumala virus (PUUV) in populations of its reservoir host species, the bank vole Myodes glareolus. In humans, the infection risks of major viral diseases are affected by the presence of helminth infections. We therefore proposed to analyse the influence of both helminth community and landscape on the prevalence of PUUV among bank vole populations in the Ardennes, a PUUV endemic area in France.
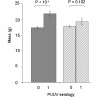
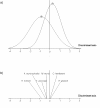
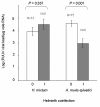
Results: Among the 313 voles analysed, 37 had anti-PUUV antibodies. Twelve gastro-intestinal helminth species were recorded among all voles sampled. We showed that PUUV seroprevalence strongly increased with age or sexual maturity, especially in the northern forests (massif des Ardennes). The helminth community structure significantly differed between this part and the woods or hedgerows of the southern cretes pre-ardennaises. Using PUUV RNA quantification, we identified significant coinfections between PUUV and gastro-intestinal helminths in the northern forests only. More specifically, PUUV infection was positively associated with the presence of Heligmosomum mixtum, and in a lesser extent, Aonchotheca muris-sylvatici. The viral load of PUUV infected individuals tended to be higher in voles coinfected with H. mixtum. It was significantly lower in voles coinfected with A. muris-sylvatici, reflecting the influence of age on these latter infections.
Conclusions: This is the first study to emphasize hantavirus--helminth coinfections in natural populations. It also highlights the importance to consider landscape when searching for such associations. We have shown that landscape characteristics strongly influence helminth community structure as well as PUUV distribution. False associations might therefore be evidenced if geographic patterns of helminths or PUUV repartition are not previously identified. Moreover, our work revealed that interactions between helminths and landscape enhance/deplete the occurrence of coinfections between PUUV and H. mixtum or A. muris-sylvatici. Further experimental analyses and long-term individual surveys are now required to confirm these correlative results, and to ascertain the causal links between helminth and PUUV infection risks.
Figures
References
-
- Gavrilovskaya IN, Apekina NS, Bernshtein AD, Demina VT, Okulova NM, Myasnikov YA, Chumakov MP. Pathogenesis of hemorrhagic fever with renal syndrome virus infection and mode of horizontal transmission of hantavirus in bank voles. Arch Virol. 1990. pp. S57–S62.
-
- Brummer-Korvenkontio M, Vaheri A, Hovi T, von Bonsdorff CH, Vuorimies J, Manni T, Penttinen K, Oker-Blom N, Lahdevirta J. Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis. 1980;141:131–134. - PubMed

