Malaria infection by sporozoite challenge induces high functional antibody titres against blood stage antigens after a DNA prime, poxvirus boost vaccination strategy in Rhesus macaques
- PMID: 21303498
- PMCID: PMC3046915
- DOI: 10.1186/1475-2875-10-29
Malaria infection by sporozoite challenge induces high functional antibody titres against blood stage antigens after a DNA prime, poxvirus boost vaccination strategy in Rhesus macaques
Abstract
Background: A DNA prime, poxvirus (COPAK) boost vaccination regime with four antigens, i.e. a combination of two Plasmodium knowlesi sporozoite (csp/ssp2) and two blood stage (ama1/msp142) genes, leads to self-limited parasitaemia in 60% of rhesus monkeys and survival from an otherwise lethal infection with P. knowlesi. In the present study, the role of the blood stage antigens in protection was studied in depth, focusing on antibody formation against the blood stage antigens and the functionality thereof.
Methods: Rhesus macaques were immunized with the four-component vaccine and subsequently challenged i.v. with 100 P. knowlesi sporozoites. During immunization and challenge, antibody titres against the two blood stage antigens were determined, as well as the in vitro growth inhibition capacity of those antibodies. Antigen reversal experiments were performed to determine the relative contribution of antibodies against each of the two blood stage antigens to the inhibition.
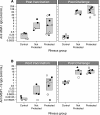
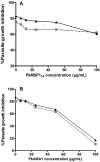
Results: After vaccination, PkAMA1 and PkMSP1₁₉ antibody titres in vaccinated animals were low, which was reflected in low levels of inhibition by these antibodies as determined by in vitro inhibition assays. Interestingly, after sporozoite challenge antibody titres against blood stage antigens were boosted over 30-fold in both protected and not protected animals. The in vitro inhibition levels increased to high levels (median inhibitions of 59% and 56% at 6 mg/mL total IgG, respectively). As growth inhibition levels were not significantly different between protected and not protected animals, the ability to control infection appeared cannot be explained by GIA levels. Judged by in vitro antigen reversal growth inhibition assays, over 85% of the inhibitory activity of these antibodies was directed against PkAMA1.
Conclusions: This is the first report that demonstrates that a DNA prime/poxvirus boost vaccination regimen induces low levels of malaria parasite growth inhibitory antibodies, which are boosted to high levels upon challenge. No association could, however, be established between the levels of inhibitory capacity in vitro and protection, either after vaccination or after challenge.
Figures

Similar articles
-
Sterile protection against Plasmodium knowlesi in rhesus monkeys from a malaria vaccine: comparison of heterologous prime boost strategies.PLoS One. 2009 Aug 10;4(8):e6559. doi: 10.1371/journal.pone.0006559. PLoS One. 2009. PMID: 19668343 Free PMC article.
-
Protection of rhesus monkeys by a DNA prime/poxvirus boost malaria vaccine depends on optimal DNA priming and inclusion of blood stage antigens.PLoS One. 2007 Oct 24;2(10):e1063. doi: 10.1371/journal.pone.0001063. PLoS One. 2007. PMID: 17957247 Free PMC article.
-
Protection of rhesus macaques against lethal Plasmodium knowlesi malaria by a heterologous DNA priming and poxvirus boosting immunization regimen.Infect Immun. 2002 Aug;70(8):4329-35. doi: 10.1128/IAI.70.8.4329-4335.2002. Infect Immun. 2002. PMID: 12117942 Free PMC article.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
-
Protective antigens of bloodstage Plasmodium knowlesi parasites.Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):159-69. doi: 10.1098/rstb.1984.0116. Philos Trans R Soc Lond B Biol Sci. 1984. PMID: 6151680 Review.
Cited by
-
Vaccination with Plasmodium knowlesi AMA1 formulated in the novel adjuvant co-vaccine HT™ protects against blood-stage challenge in rhesus macaques.PLoS One. 2011;6(5):e20547. doi: 10.1371/journal.pone.0020547. Epub 2011 May 31. PLoS One. 2011. PMID: 21655233 Free PMC article.
-
Using infective mosquitoes to challenge monkeys with Plasmodium knowlesi in malaria vaccine studies.Malar J. 2014 Jun 3;13:215. doi: 10.1186/1475-2875-13-215. Malar J. 2014. PMID: 24893777 Free PMC article.
-
Activity of Plasmodium vivax promoter elements in Plasmodium knowlesi, and a centromere-containing plasmid that expresses NanoLuc throughout the parasite life cycle.Malar J. 2021 Jun 5;20(1):247. doi: 10.1186/s12936-021-03773-4. Malar J. 2021. PMID: 34090438 Free PMC article.
-
Rhesus macaque and mouse models for down-selecting circumsporozoite protein based malaria vaccines differ significantly in immunogenicity and functional outcomes.Malar J. 2017 Mar 13;16(1):115. doi: 10.1186/s12936-017-1766-3. Malar J. 2017. PMID: 28288639 Free PMC article.
-
Admixture in Humans of Two Divergent Plasmodium knowlesi Populations Associated with Different Macaque Host Species.PLoS Pathog. 2015 May 28;11(5):e1004888. doi: 10.1371/journal.ppat.1004888. eCollection 2015 May. PLoS Pathog. 2015. PMID: 26020959 Free PMC article.
References
-
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. - DOI - PubMed
-
- Thomas AW, Slierendregt B, Mons B, Druilhe P. Chimpanzees and supporting models in the study of malaria pre-erythrocytic stages. Mem Inst Oswaldo Cruz. 1994;89(Suppl 2):111–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

