The design and development of fluorescent nano-optodes for in vivo glucose monitoring
- PMID: 21303627
- PMCID: PMC3045232
- DOI: 10.1177/193229681100500110
The design and development of fluorescent nano-optodes for in vivo glucose monitoring
Abstract
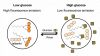
Background: The advent of fluorescent nanosensors has enabled intracellular monitoring of several physiological analytes, which was previously not possible with molecular dyes or other invasive techniques. We have extended the capability of these sensors to include the detection of small molecules with the development of glucose-sensitive nano-optodes. Herein, we discuss the design and development of glucose-sensitive nano-optodes, which have been proven functional both in vitro and in vivo.
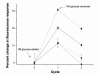
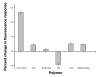
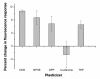
Methods: Throughout the design process, each of the sensor formulations was evaluated based on their response to changes in glucose levels. The percent change in signal, sensor reversibility, and the overall fluorescence intensity were the specific parameters used to assess each formulation.
Results: A hydrophobic boronic acid was selected that yielded a fully reversible fluorescence response to glucose in accordance with the sensor mechanism. The change in fluorescence signal in response to glucose was approximately 11%. The use of different additives or chromophores did not improve the response; however, modifications to the plasticized polymeric membrane extended sensor lifetime.
Conclusions: Sensors were developed that yielded a dynamic response to glucose and through further modification of the components, sensor lifetime was improved. By following specific design criteria for the macrosensors, the sensors were miniaturized into nano-optodes that track changes in glucose levels in vivo.
© 2010 Diabetes Technology Society.
Figures
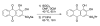
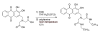


References
-
- Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108(2):814–825. - PubMed
-
- Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev. 2008;108(7):2482–2505. - PubMed
-
- McGarraugh G. The chemistry of commercial continuous glucose monitors. Diabetes Technol Ther. 2009;11(Suppl 1):S17–S24. - PubMed
-
- Pickup JC, Hussain F, Evans ND, Sachedina N. In vivo glucose monitoring: the clinical reality and promise. Biosens Bioelectron. 2005;20(10):1897–1902. - PubMed
-
- Klueh U, Liu Z, Cho B, Ouyang T, Feldman B, Henning TP, Kaur M, Kreutzer D. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther. 2006;8(3):412–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

