Identification of new batrachotoxin-sensing residues in segment IIIS6 of the sodium channel
- PMID: 21303907
- PMCID: PMC3075662
- DOI: 10.1074/jbc.M110.208496
Identification of new batrachotoxin-sensing residues in segment IIIS6 of the sodium channel
Abstract
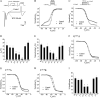
Ion permeation through voltage-gated sodium channels is modulated by various drugs and toxins. The atomistic mechanisms of action of many toxins are poorly understood. A steroidal alkaloid batrachotoxin (BTX) causes persistent channel activation by inhibiting inactivation and shifting the voltage dependence of activation to more negative potentials. Traditionally, BTX is considered to bind at the channel-lipid interface and allosterically modulate the ion permeation. However, amino acid residues critical for BTX action are found in the inner helices of all four repeats, suggesting that BTX binds in the pore. In the octapeptide segment IFGSFFTL in IIIS6 of a cockroach sodium channel BgNa(V), besides Ser_3i15 and Leu_3i19, which correspond to known BTX-sensing residues of mammalian sodium channels, we found that Gly_3i14 and Phe_3i16 are critical for BTX action. Using these data along with published data as distance constraints, we docked BTX in the Kv1.2-based homology model of the open BgNa(V) channel. We arrived at a model in which BTX adopts a horseshoe conformation with the horseshoe plane normal to the pore axis. The BTX ammonium group is engaged in cation-π interactions with Phe_3i16 and BTX moieties interact with known BTX-sensing residues in all four repeats. Oxygen atoms at the horseshoe inner surface constitute a transient binding site for permeating cations, whereas the bulky BTX molecule would resist the pore closure, thus causing persistent channel activation. Our study reinforces the concept that steroidal sodium channel agonists bind in the inner pore of sodium channels and elaborates the atomistic mechanism of BTX action.
Figures
Similar articles
-
Batrachotoxin acts as a stent to hold open homotetrameric prokaryotic voltage-gated sodium channels.J Gen Physiol. 2019 Feb 4;151(2):186-199. doi: 10.1085/jgp.201812278. Epub 2018 Dec 26. J Gen Physiol. 2019. PMID: 30587506 Free PMC article.
-
Batrachotoxin, pyrethroids, and BTG 502 share overlapping binding sites on insect sodium channels.Mol Pharmacol. 2011 Sep;80(3):426-33. doi: 10.1124/mol.111.072504. Epub 2011 Jun 16. Mol Pharmacol. 2011. PMID: 21680776 Free PMC article.
-
Identification of a cluster of residues in transmembrane segment 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides.Biochem J. 2009 Apr 15;419(2):377-85. doi: 10.1042/BJ20082082. Biochem J. 2009. PMID: 19154185 Free PMC article.
-
[Steroidal alkaloid batrachotoxin--instrument for studying voltage-regulated sodium channels].Neirofiziologiia. 1985;17(3):409-22. Neirofiziologiia. 1985. PMID: 2410800 Review. Russian.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
Cited by
-
Batrachotoxin acts as a stent to hold open homotetrameric prokaryotic voltage-gated sodium channels.J Gen Physiol. 2019 Feb 4;151(2):186-199. doi: 10.1085/jgp.201812278. Epub 2018 Dec 26. J Gen Physiol. 2019. PMID: 30587506 Free PMC article.
-
Animal toxins influence voltage-gated sodium channel function.Handb Exp Pharmacol. 2014;221:203-29. doi: 10.1007/978-3-642-41588-3_10. Handb Exp Pharmacol. 2014. PMID: 24737238 Free PMC article. Review.
-
Evidence that toxin resistance in poison birds and frogs is not rooted in sodium channel mutations and may rely on "toxin sponge" proteins.J Gen Physiol. 2021 Sep 6;153(9):e202112872. doi: 10.1085/jgp.202112872. Epub 2021 Aug 5. J Gen Physiol. 2021. PMID: 34351379 Free PMC article.
-
Peptide toxins that target vertebrate voltage-gated sodium channels underly the painful stings of harvester ants.J Biol Chem. 2024 Jan;300(1):105577. doi: 10.1016/j.jbc.2023.105577. Epub 2023 Dec 16. J Biol Chem. 2024. PMID: 38110035 Free PMC article.
-
Hydrophobic Drug/Toxin Binding Sites in Voltage-Dependent K+ and Na+ Channels.Front Pharmacol. 2020 May 15;11:735. doi: 10.3389/fphar.2020.00735. eCollection 2020. Front Pharmacol. 2020. PMID: 32499709 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

