The intrinsically disordered nuclear localization signal and phosphorylation segments distinguish the membrane affinity of two cytidylyltransferase isoforms
- PMID: 21303909
- PMCID: PMC3069438
- DOI: 10.1074/jbc.M110.201715
The intrinsically disordered nuclear localization signal and phosphorylation segments distinguish the membrane affinity of two cytidylyltransferase isoforms
Abstract
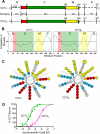
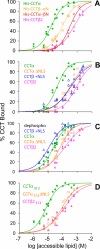
Membrane phosphatidylcholine homeostasis is maintained in part by a sensing device in the key regulatory enzyme, CTP:phosphocholine cytidylyltransferase (CCT). CCT responds to decreases in membrane phosphatidylcholine content by reversible membrane binding and activation. Two prominent isoforms, CCTα and -β2, have nearly identical catalytic domains and very similar membrane binding amphipathic helical (M) domains but have divergent and structurally disordered N-terminal (N) and C-terminal phosphorylation (P) regions. We found that the binding affinity of purified CCTβ2 for anionic membranes was weaker than CCTα by more than an order of magnitude. Using chimeric CCTs, insertion/deletion mutants, and truncated CCTs, we show that the stronger affinity of CCTα can be attributed in large part to the electrostatic membrane binding function of the polybasic nuclear localization signal (NLS) motif, present in the unstructured N-terminal segment of CCTα but lacking in CCTβ2. The membrane partitioning of CCTβ2 in cells enriched with the lipid activator, oleic acid, was also weaker than that of CCTα and was elevated by incorporation of the NLS motif. Thus, the polybasic NLS can function as a secondary membrane binding motif not only in vitro but in the context of cell membranes. A comparison of phosphorylated, dephosphorylated, and region P-truncated forms showed that the in vitro membrane affinity of CCTβ2 is more sensitive than CCTα to phosphorylation status, which antagonizes membrane binding of both isoforms. These data provide a model wherein the primary membrane binding motif, an amphipathic helical domain, works in collaboration with other intrinsically disordered segments that modulate membrane binding strength. The NLS reinforces, whereas the phosphorylated tail antagonizes the attraction of domain M for anionic membranes.
Figures

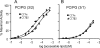
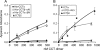
Similar articles
-
Lipid activation of CTP: phosphocholine cytidylyltransferase alpha: characterization and identification of a second activation domain.Biochemistry. 2001 Jan 16;40(2):494-503. doi: 10.1021/bi002140r. Biochemistry. 2001. PMID: 11148044
-
Distribution of CTP:phosphocholine cytidylyltransferase (CCT) isoforms. Identification of a new CCTbeta splice variant.J Biol Chem. 1999 Sep 17;274(38):26992-7001. doi: 10.1074/jbc.274.38.26992. J Biol Chem. 1999. PMID: 10480912
-
Disease-linked mutations in the phosphatidylcholine regulatory enzyme CCTα impair enzymatic activity and fold stability.J Biol Chem. 2019 Feb 1;294(5):1490-1501. doi: 10.1074/jbc.RA118.006457. Epub 2018 Dec 17. J Biol Chem. 2019. PMID: 30559292 Free PMC article.
-
CTP:phosphocholine cytidylyltransferase: insights into regulatory mechanisms and novel functions.Biochem Biophys Res Commun. 1999 Apr 21;257(3):643-50. doi: 10.1006/bbrc.1999.0512. Biochem Biophys Res Commun. 1999. PMID: 10208837 Review.
-
Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization.Trends Biochem Sci. 2000 Sep;25(9):441-7. doi: 10.1016/s0968-0004(00)01625-x. Trends Biochem Sci. 2000. PMID: 10973058 Review.
Cited by
-
Differential dephosphorylation of CTP:phosphocholine cytidylyltransferase upon translocation to nuclear membranes and lipid droplets.Mol Biol Cell. 2020 May 1;31(10):1047-1059. doi: 10.1091/mbc.E20-01-0014. Epub 2020 Mar 18. Mol Biol Cell. 2020. PMID: 32186954 Free PMC article.
-
Remodeling of the interdomain allosteric linker upon membrane binding of CCTα pulls its active site close to the membrane surface.J Biol Chem. 2019 Oct 18;294(42):15531-15543. doi: 10.1074/jbc.RA119.009850. Epub 2019 Sep 4. J Biol Chem. 2019. PMID: 31488548 Free PMC article.
-
Phosphorylation of Human Choline Kinase Beta by Protein Kinase A: Its Impact on Activity and Inhibition.PLoS One. 2016 May 5;11(5):e0154702. doi: 10.1371/journal.pone.0154702. eCollection 2016. PLoS One. 2016. PMID: 27149373 Free PMC article.
-
An auto-inhibitory helix in CTP:phosphocholine cytidylyltransferase hijacks the catalytic residue and constrains a pliable, domain-bridging helix pair.J Biol Chem. 2018 May 4;293(18):7070-7084. doi: 10.1074/jbc.RA118.002053. Epub 2018 Mar 8. J Biol Chem. 2018. PMID: 29519816 Free PMC article.
-
Identification of a nuclear localization signal in the Plasmodium falciparum CTP: phosphocholine cytidylyltransferase enzyme.Sci Rep. 2020 Nov 12;10(1):19739. doi: 10.1038/s41598-020-76829-1. Sci Rep. 2020. PMID: 33184408 Free PMC article.
References
-
- Vance J. E. (1998) Trends Biochem. Sci. 23, 423–428 - PubMed
-
- Cornell R. B., Northwood I. C. (2000) Trends Biochem. Sci. 25, 441–447 - PubMed
-
- Lykidis A., Murti K. G., Jackowski S. (1998) J. Biol. Chem. 273, 14022–14029 - PubMed
-
- Karim M., Jackson P., Jackowski S. (2003) Biochim. Biophys. Acta 1633, 1–12 - PubMed
-
- Lykidis A., Baburina I., Jackowski S. (1999) J. Biol. Chem. 274, 26992–27001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

