Regulation of transforming growth factor-β1-dependent integrin β6 expression by p38 mitogen-activated protein kinase in bile duct epithelial cells
- PMID: 21303922
- PMCID: PMC3083106
- DOI: 10.1124/jpet.110.177337
Regulation of transforming growth factor-β1-dependent integrin β6 expression by p38 mitogen-activated protein kinase in bile duct epithelial cells
Abstract
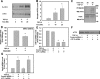
Bile duct epithelial cells (BDECs) contribute to liver fibrosis by expressing αVβ6 integrin, a critical activator of latent transforming growth factor β (TGF-β). β6 integrin (Itgβ6) mRNA induction and αVβ6 integrin expression in BDECs are partially TGF-β-dependent. However, the signaling pathways required for TGF-β-dependent Itgβ6 mRNA induction in BDECs are not known. We tested the hypothesis that the p38 mitogen-activated protein kinase (MAPK) signaling pathway contributes to TGF-β1 induction of Itgβ6 mRNA by activating SMAD and activator protein 1 (AP-1) transcription factors. Pretreatment of transformed human BDECs (MMNK-1 cells) with two different p38 MAPK inhibitors, but not a control compound, inhibited TGF-β1 induction of Itgβ6 mRNA. Inhibition of p38 also reduced TGF-β1 activation of a SMAD-dependent reporter construct. Expression of a dominant-negative SMAD3 (SMAD3ΔC) significantly reduced TGF-β1-induced Itgβ6 mRNA expression. Expression of JunB mRNA, but not other AP-1 proteins, increased in TGF-β1-treated MMNK-1 cells, and induction of JunB expression was p38-dependent. Consistent with a requirement for de novo induction of JunB protein, cycloheximide pretreatment inhibited TGF-β1 induction of Itgβ6 mRNA. Expression of a dominant-negative AP-1 mutant (TAM67) also inhibited TGF-β1 induction of Itgβ6 mRNA. Overall, the results suggest that p38 contributes to TGF-β1-induced Itgβ6 mRNA expression in MMNK-1 cells by regulating activation of both SMAD and AP-1 transcription factors.
Figures


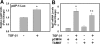

Similar articles
-
Lipopolysaccharide enhances transforming growth factor β1-induced platelet-derived growth factor-B expression in bile duct epithelial cells.J Gastroenterol Hepatol. 2012 Apr;27(4):714-21. doi: 10.1111/j.1440-1746.2011.06941.x. J Gastroenterol Hepatol. 2012. PMID: 22004089 Free PMC article.
-
ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-beta1 autoinduction in proximal tubular epithelial cells.Am J Pathol. 2006 Oct;169(4):1282-93. doi: 10.2353/ajpath.2006.050921. Am J Pathol. 2006. PMID: 17003485 Free PMC article.
-
Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways.J Cell Biochem. 2005 Aug 1;95(5):918-31. doi: 10.1002/jcb.20458. J Cell Biochem. 2005. PMID: 15861394
-
Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat.J Mol Med (Berl). 2006 Nov;84(11):975-83. doi: 10.1007/s00109-006-0090-0. Epub 2006 Aug 19. J Mol Med (Berl). 2006. PMID: 16924465
-
Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-beta1-induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro.Lung. 2006 Jan-Feb;184(1):33-42. doi: 10.1007/s00408-005-2560-5. Lung. 2006. PMID: 16598650
Cited by
-
ETS2 promotes epithelial-to-mesenchymal transition in renal fibrosis by targeting JUNB transcription.Lab Invest. 2020 Mar;100(3):438-453. doi: 10.1038/s41374-019-0331-9. Epub 2019 Oct 22. Lab Invest. 2020. PMID: 31641227
-
The clinical significance and underlying correlation of pStat-3 and integrin αvβ6 expression in gallbladder cancer.Oncotarget. 2017 Mar 21;8(12):19467-19477. doi: 10.18632/oncotarget.14444. Oncotarget. 2017. PMID: 28061445 Free PMC article.
-
Modulation of αVβ6 integrin in osteoarthritis-related synovitis and the interaction with VTN(381-397 a.a.) competing for TGF-β1 activation.Exp Mol Med. 2021 Feb;53(2):210-222. doi: 10.1038/s12276-021-00558-2. Epub 2021 Feb 1. Exp Mol Med. 2021. PMID: 33526813 Free PMC article.
-
Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro.J Cell Sci. 2013 May 15;126(Pt 10):2164-75. doi: 10.1242/jcs.111302. Epub 2013 Mar 22. J Cell Sci. 2013. PMID: 23525012 Free PMC article.
-
From the Cover: Coagulation-Driven Hepatic Fibrosis Requires Protease Activated Receptor-1 (PAR-1) in a Mouse Model of TCDD-Elicited Steatohepatitis.Toxicol Sci. 2016 Dec;154(2):381-391. doi: 10.1093/toxsci/kfw175. Epub 2016 Sep 9. Toxicol Sci. 2016. PMID: 27613713 Free PMC article.
References
-
- Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W. (1995) Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 108:2241–2251 - PubMed
-
- Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. (1993) Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene 8:877–886 - PubMed
-
- Brown PH, Chen TK, Birrer MJ. (1994) Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene 9:791–799 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

