Understanding ribosome assembly: the structure of in vivo assembled immature 30S subunits revealed by cryo-electron microscopy
- PMID: 21303937
- PMCID: PMC3062180
- DOI: 10.1261/rna.2509811
Understanding ribosome assembly: the structure of in vivo assembled immature 30S subunits revealed by cryo-electron microscopy
Abstract
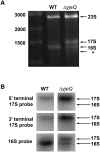
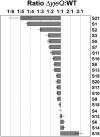
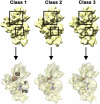
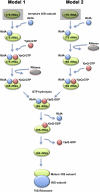
Four decades after early in vitro assembly studies demonstrated that ribosome assembly is a controlled process, our understanding of ribosome assembly is still incomplete. Just as structure determination has been so important to understanding ribosome function, so too will it be critical to sorting out the assembly process. Here, we used a viable deletion in the yjeQ gene, a recognized ribosome assembly factor, to isolate and structurally characterize immature 30S subunits assembled in vivo. These small ribosome subunits contained unprocessed 17S rRNA and lacked some late ribosomal proteins. Cryo-electron microscopy reconstructions revealed that the presence of precursor sequences in the rRNA induces a severe distortion in the 3' minor domain of the subunit involved in the decoding of mRNA and interaction with the large ribosome subunit. These findings suggest that rRNA processing events induce key local conformational changes directing the structure toward the mature assembly. We concluded that rRNA processing, folding, and the entry of tertiary r-proteins are interdependent events in the late stages of 30S subunit assembly. In addition, we demonstrate how studies of emerging assembly factors in ribosome biogenesis can help to elucidate the path of subunit assembly in vivo.
Figures

Similar articles
-
Escherichia coli rimM and yjeQ null strains accumulate immature 30S subunits of similar structure and protein complement.RNA. 2013 Jun;19(6):789-802. doi: 10.1261/rna.037523.112. Epub 2013 Apr 23. RNA. 2013. PMID: 23611982 Free PMC article.
-
Structural insights into the assembly of the 30S ribosomal subunit in vivo: functional role of S5 and location of the 17S rRNA precursor sequence.Protein Cell. 2014 May;5(5):394-407. doi: 10.1007/s13238-014-0044-1. Epub 2014 Mar 28. Protein Cell. 2014. PMID: 24671761 Free PMC article.
-
The cryo-EM structure of YjeQ bound to the 30S subunit suggests a fidelity checkpoint function for this protein in ribosome assembly.Proc Natl Acad Sci U S A. 2017 Apr 25;114(17):E3396-E3403. doi: 10.1073/pnas.1618016114. Epub 2017 Apr 10. Proc Natl Acad Sci U S A. 2017. PMID: 28396444 Free PMC article.
-
Protein Assistants of Small Ribosomal Subunit Biogenesis in Bacteria.Microorganisms. 2022 Mar 30;10(4):747. doi: 10.3390/microorganisms10040747. Microorganisms. 2022. PMID: 35456798 Free PMC article. Review.
-
Assembly of bacterial ribosomes.Annu Rev Biochem. 2011;80:501-26. doi: 10.1146/annurev-biochem-062608-160432. Annu Rev Biochem. 2011. PMID: 21529161 Review.
Cited by
-
Purification and Characterization of Authentic 30S Ribosomal Precursors Induced by Heat Shock.Int J Mol Sci. 2023 Feb 9;24(4):3491. doi: 10.3390/ijms24043491. Int J Mol Sci. 2023. PMID: 36834906 Free PMC article.
-
Quantitative mining of compositional heterogeneity in cryo-EM datasets of ribosome assembly intermediates.Structure. 2022 Apr 7;30(4):498-509.e4. doi: 10.1016/j.str.2021.12.005. Epub 2022 Jan 5. Structure. 2022. PMID: 34990602 Free PMC article.
-
Escherichia coli rimM and yjeQ null strains accumulate immature 30S subunits of similar structure and protein complement.RNA. 2013 Jun;19(6):789-802. doi: 10.1261/rna.037523.112. Epub 2013 Apr 23. RNA. 2013. PMID: 23611982 Free PMC article.
-
Carbohydrate-based drugs launched during 2000-2021.Acta Pharm Sin B. 2022 Oct;12(10):3783-3821. doi: 10.1016/j.apsb.2022.05.020. Epub 2022 May 23. Acta Pharm Sin B. 2022. PMID: 36213536 Free PMC article. Review.
-
Emerging Quantitative Biochemical, Structural, and Biophysical Methods for Studying Ribosome and Protein-RNA Complex Assembly.Biomolecules. 2023 May 19;13(5):866. doi: 10.3390/biom13050866. Biomolecules. 2023. PMID: 37238735 Free PMC article. Review.
References
-
- Aebi U, Pollard TD 1987. A glow discharge unit to render electron microscope grids and other surfaces hydrophilic. J Electron Microsc Tech 7: 29–33 - PubMed
-
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289: 905–920 - PubMed
-
- Borovinskaya MA, Pai RD, Zhang W, Schuwirth BS, Holton JM, Hirokawa G, Kaji H, Kaji A, Cate JH 2007. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat Struct Mol Biol 14: 727–732 - PubMed
-
- Brown ED 2005. Conserved P-loop GTPases of unknown function in bacteria: an emerging and vital ensemble in bacterial physiology. Biochem Cell Biol 83: 738–746 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
