GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor alpha (ERα) phosphorylation signals
- PMID: 21303939
- PMCID: PMC3060628
- DOI: 10.1210/en.2010-1368
GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor alpha (ERα) phosphorylation signals
Abstract
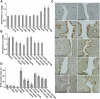
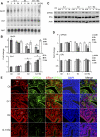
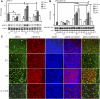
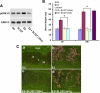
Although estradiol-17β (E2)-regulated early and late phase uterine responses have been well defined, the molecular mechanisms linking the phases remain poorly understood. We have previously shown that E2-regulated early signals mediate cross talk with estrogen receptor (ER)-α to elicit uterine late growth responses. G protein-coupled receptor (GPR30) has been implicated in early nongenomic signaling mediated by E2, although its role in E2-dependent uterine biology is unclear. Using selective activation of GPR30 by G-1, we show here a new function of GPR30 in regulating early signaling events, including the inhibition of ERK1/2 and ERα (Ser118) phosphorylation signals and perturbation of growth regulation under the direction of E2 in the mouse uterus. We observed that GPR30 primarily localizes in the uterine epithelial cells, and its activation alters gene expression and mediates inhibition of ERK1/2 and ERα (Ser118) phosphorylation signals in the stromal compartment, suggesting a paracrine signaling is involved. Importantly, viral-driven manipulation of GPR30 or pharmacological inhibition of ERK1/2 activation effectively alters E2-dependent uterine growth responses. Overall, GPR30 is a negative regulator of ERα-dependent uterine growth in response to E2. Our work has uncovered a novel GPR30-regulated inhibitory event, which may be physiologically relevant in both normal and pathological situations to negatively balance ERα-dependent uterine growth regulatory functions induced by E2.
Figures


Similar articles
-
G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells.Cancer Res. 2007 Feb 15;67(4):1859-66. doi: 10.1158/0008-5472.CAN-06-2909. Cancer Res. 2007. PMID: 17308128
-
Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling.Mol Endocrinol. 2010 Apr;24(4):709-21. doi: 10.1210/me.2009-0317. Epub 2010 Mar 2. Mol Endocrinol. 2010. PMID: 20197310 Free PMC article.
-
The sequence Pro295-Thr311 of the hinge region of oestrogen receptor α is involved in ERK1/2 activation via GPR30 in leiomyoma cells.Biochem J. 2015 Nov 15;472(1):97-109. doi: 10.1042/BJ20150744. Epub 2015 Sep 14. Biochem J. 2015. PMID: 26371374
-
ERK1/2-RSK2 Signaling in Regulation of ERα-Mediated Responses.Endocrinology. 2022 Sep 1;163(9):bqac106. doi: 10.1210/endocr/bqac106. Endocrinology. 2022. PMID: 35880639 Free PMC article. Review.
-
Mechanisms of estrogen signaling and gene expression via GPR30.Mol Cell Endocrinol. 2009 Sep 24;308(1-2):32-8. doi: 10.1016/j.mce.2009.03.026. Epub 2009 Apr 15. Mol Cell Endocrinol. 2009. PMID: 19464786 Free PMC article. Review.
Cited by
-
Role of Estrogen Receptor β, G-Protein Coupled Estrogen Receptor and Estrogen-Related Receptors in Endometrial and Ovarian Cancer.Cancers (Basel). 2023 May 20;15(10):2845. doi: 10.3390/cancers15102845. Cancers (Basel). 2023. PMID: 37345182 Free PMC article. Review.
-
What have we learned about GPER function in physiology and disease from knockout mice?J Steroid Biochem Mol Biol. 2015 Sep;153:114-26. doi: 10.1016/j.jsbmb.2015.06.014. Epub 2015 Jul 16. J Steroid Biochem Mol Biol. 2015. PMID: 26189910 Free PMC article. Review.
-
Estrogen, Angiogenesis, Immunity and Cell Metabolism: Solving the Puzzle.Int J Mol Sci. 2018 Mar 15;19(3):859. doi: 10.3390/ijms19030859. Int J Mol Sci. 2018. PMID: 29543707 Free PMC article. Review.
-
Mammary epithelial cell phenotype disruption in vitro and in vivo through ERalpha36 overexpression.PLoS One. 2017 Mar 16;12(3):e0173931. doi: 10.1371/journal.pone.0173931. eCollection 2017. PLoS One. 2017. PMID: 28301550 Free PMC article.
-
The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function.Vascul Pharmacol. 2011 Jul-Sep;55(1-3):17-25. doi: 10.1016/j.vph.2011.06.003. Epub 2011 Jul 5. Vascul Pharmacol. 2011. PMID: 21742056 Free PMC article. Review.
References
-
- Couse JF, Korach KS. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 - PubMed
-
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. 1991. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses' Health Study. N Engl J Med 325:756–762 - PubMed
-
- McDonnell DP, Norris JD. 2002. Connections and regulation of the human estrogen receptor. Science 296:1642–1644 - PubMed
-
- Tsai MJ, O'Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 - PubMed
-
- Beato M, Herrlich P, Schütz G. 1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

