Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation
- PMID: 21303954
- PMCID: PMC3060634
- DOI: 10.1210/en.2010-1121
Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation
Abstract
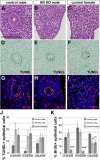
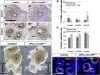
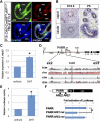
The epididymis is a male accessory organ and functions for sperm maturation and storage under the control of androgen. The development of the epididymis is also androgen dependent. The Wolffian duct (WD), anlagen of the epididymis, is formed in both male and female embryos; however, it is stabilized only in male embryos by testicular androgen. Androgen drives subsequent differentiation of the WD into the epididymis. Although the essential roles of androgen in WD masculinization and epididymal function have been established, little is known about cellular events regulated precisely by androgen signaling during these processes. It is also unclear whether androgen signaling, especially in the epithelia, has further function for epididymal epithelial cell differentiation. In this study we examined the cellular death and proliferation controlled by androgen signaling via the androgen receptor (AR) in WD stabilization. Analyses using AR knockout mice revealed that androgen signaling inhibits epithelial cell death in this process. Analysis of AP2α-Cre;AR(flox/Y) mice, in which AR function is deleted in the WD epithelium, revealed that epithelial AR is not required for the WD stabilization but is required for epithelial cell differentiation in the epididymis. Specifically, loss of epithelial AR significantly reduced expression of p63 that is essential for differentiation of basal cells in the epididymal epithelium. We also interrogated the possibility of regulation of the p63 gene (Trp63) by AR in vitro and found that p63 is a likely direct target of AR regulation.
Figures



References
-
- Jones RC. 2002. Evolution of the vertebrate epididymis. In: Robaire B, Hinton BT. ed. The epididymis. New York: Kluwer Academic/Plenum Publishers; 11–33
-
- Hinton BT, Palladino MA. 1995. Epididymal epithelium: its contribution to the formation of a luminal fluid microenvironment. Microsc Res Tech 30:67–81 - PubMed
-
- Zhou CX, Zhang YL, Xiao L, Zheng M, Leung KM, Chan MY, Lo PS, Tsang LL, Wong HY, Ho LS, Chung YW, Chan HC. 2004. An epididymis-specific β-defensin is important for the initiation of sperm maturation. Nat Cell Biol 6:458–464 - PubMed
-
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. 2003. Mouse urogenital development: a practical approach. Differentiation 71:402–413 - PubMed
-
- Jost A. 1970. Hormonal factors in the sex differentiation of the mammalian foetus. Philos Trans R Soc Lond B Biol Sci 259:119–130 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

