Transgenic overexpression of the extra-large Gsα variant XLαs enhances Gsα-mediated responses in the mouse renal proximal tubule in vivo
- PMID: 21303955
- PMCID: PMC3060637
- DOI: 10.1210/en.2010-1034
Transgenic overexpression of the extra-large Gsα variant XLαs enhances Gsα-mediated responses in the mouse renal proximal tubule in vivo
Abstract
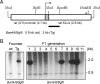
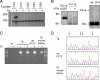
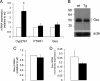
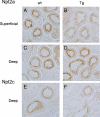
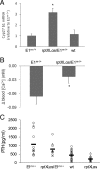
XLαs, a variant of the stimulatory G protein α-subunit (Gsα), can mediate receptor-activated cAMP generation and, thus, mimic the actions of Gsα in transfected cells. However, it remains unknown whether XLαs can act in a similar manner in vivo. We have now generated mice with ectopic transgenic expression of rat XLαs in the renal proximal tubule (rptXLαs mice), where Gsα mediates most actions of PTH. Western blots and quantitative RT-PCR showed that, while Gsα and type-1 PTH receptor levels were unaltered, protein kinase A activity and 25-hydroxyvitamin D 1-α-hydroxylase (Cyp27b1) mRNA levels were significantly higher in renal proximal tubules of rptXLαs mice than wild-type littermates. Immunohistochemical analysis of kidney sections showed that the sodium-phosphate cotransporter type 2a was modestly reduced in brush border membranes of male rptXLαs mice compared to gender-matched controls. Serum calcium, phosphorus, and 1,25 dihydroxyvitamin D were within the normal range, but serum PTH was ∼30% lower in rptXLαs mice than in controls (152 ± 16 vs. 222 ± 41 pg/ml; P < 0.05). After crossing the rptXLαs mice to mice with ablation of maternal Gnas exon 1 (E1(m-/+)), male offspring carrying both the XLαs transgene and maternal Gnas exon 1 ablation (rptXLαs/E1(m-/+)) were significantly less hypocalcemic than gender-matched E1(m-/+) littermates. Both E1(m-/+) and rptXLαs/E1(m-/+) offspring had higher serum PTH than wild-type littermates, but the degree of secondary hyperparathyroidism tended to be lower in rptXLαs/E1(m-/+) mice. Hence, transgenic XLαs expression in the proximal tubule enhanced Gsα-mediated responses, indicating that XLαs can mimic Gsα in vivo.
Figures


Similar articles
-
GNAS mutations and heterotopic ossification.Bone. 2018 Apr;109:80-85. doi: 10.1016/j.bone.2017.09.002. Epub 2017 Sep 6. Bone. 2018. PMID: 28889026 Free PMC article. Review.
-
The G protein α subunit variant XLαs promotes inositol 1,4,5-trisphosphate signaling and mediates the renal actions of parathyroid hormone in vivo.Sci Signal. 2015 Aug 25;8(391):ra84. doi: 10.1126/scisignal.aaa9953. Sci Signal. 2015. PMID: 26307011 Free PMC article.
-
Coding GNAS mutations leading to hormone resistance impair in vitro agonist- and cholera toxin-induced adenosine cyclic 3',5'-monophosphate formation mediated by human XLalphas.Endocrinology. 2006 May;147(5):2253-62. doi: 10.1210/en.2005-1487. Epub 2006 Feb 16. Endocrinology. 2006. PMID: 16484323
-
Potent constitutive cyclic AMP-generating activity of XLαs implicates this imprinted GNAS product in the pathogenesis of McCune-Albright syndrome and fibrous dysplasia of bone.Bone. 2011 Feb;48(2):312-20. doi: 10.1016/j.bone.2010.09.032. Epub 2010 Sep 29. Bone. 2011. PMID: 20887824 Free PMC article.
-
GNAS gene mutations affecting XLαs and bone health: A long neglected relationship.Clin Genet. 2023 Sep;104(3):279-286. doi: 10.1111/cge.14378. Epub 2023 May 30. Clin Genet. 2023. PMID: 37249323 Review.
Cited by
-
The N termini of a-subunit isoforms are involved in signaling between vacuolar H+-ATPase (V-ATPase) and cytohesin-2.J Biol Chem. 2013 Feb 22;288(8):5896-913. doi: 10.1074/jbc.M112.409169. Epub 2013 Jan 3. J Biol Chem. 2013. PMID: 23288846 Free PMC article.
-
Methylation defect in imprinted genes detected in patients with an Albright's hereditary osteodystrophy like phenotype and platelet Gs hypofunction.PLoS One. 2012;7(6):e38579. doi: 10.1371/journal.pone.0038579. Epub 2012 Jun 5. PLoS One. 2012. PMID: 22679513 Free PMC article.
-
Loss of XLαs (extra-large αs) imprinting results in early postnatal hypoglycemia and lethality in a mouse model of pseudohypoparathyroidism Ib.Proc Natl Acad Sci U S A. 2012 Apr 24;109(17):6638-43. doi: 10.1073/pnas.1117608109. Epub 2012 Apr 10. Proc Natl Acad Sci U S A. 2012. PMID: 22496590 Free PMC article.
-
GNAS mutations and heterotopic ossification.Bone. 2018 Apr;109:80-85. doi: 10.1016/j.bone.2017.09.002. Epub 2017 Sep 6. Bone. 2018. PMID: 28889026 Free PMC article. Review.
-
Gsα Regulates Macrophage Foam Cell Formation During Atherosclerosis.Circ Res. 2024 Mar 29;134(7):e34-e51. doi: 10.1161/CIRCRESAHA.123.323156. Epub 2024 Feb 20. Circ Res. 2024. PMID: 38375634 Free PMC article.
References
-
- Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M. 2004. Minireview: GNAS: normal and abnormal functions. Endocrinology 145:5459–5464 - PubMed
-
- Plagge A, Kelsey G, Germain-Lee EL. 2008. Physiological functions of the imprinted Gnas locus and its protein variants Galpha(s) and XLalpha(s) in human and mouse. J Endocrinol 196:193–214 - PubMed
-
- Kehlenbach RH, Matthey J, Huttner WB. 1994. XLαs is a new type of G protein. Nature [Erratum (1995) 375:253] 372:804–809 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

