Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features
- PMID: 21304001
- PMCID: PMC5317273
- DOI: 10.1158/1078-0432.CCR-10-1706
Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features
Abstract
Purpose: Seneca Valley Virus (SVV-001) is a novel naturally occurring replication-competent picornavirus with potent and selective tropism for neuroendocrine cancer cell types, including small cell lung cancer. We conducted a first-in-human, first-in-class phase I clinical trial of this agent in patients with cancers with neuroendocrine features, including small cell lung cancer.
Experimental design: Clinical evaluation of single intravenous doses in patients with cancers with neuroendocrine features was performed across five log-increments from 10(7) to 10(11) vp/kg. Toxicity, viral titers and clearance, neutralizing antibody development, and tumor response were assessed.
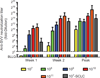
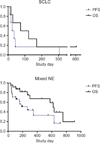
Results: A total of 30 patients were treated with SVV-001, including six with small cell carcinoma at the lowest dose of 10(7) vp/kg. SVV-001 was well tolerated, with no dose-limiting toxicities observed in any dose cohort. Viral clearance was documented in all subjects and correlated temporally with development of antiviral antibodies. Evidence of in vivo intratumoral viral replication was observed among patients with small cell carcinoma, with peak viral titers estimated to be >10(3)-fold higher than the administered dose. One patient with previously progressive chemorefractory small cell lung cancer remained progression-free for 10 months after SVV-001 administration, and is alive over 3 years after treatment.
Conclusions: Intravenous SVV-001 administration in patients is well tolerated at doses up to 10(11) vp/kg, with predictable viral clearance kinetics, intratumoral viral replication, and evidence of antitumor activity in patients with small cell lung cancer. Phase II clinical evaluation in small cell lung cancer is warranted, and has been initiated.
©2011 AACR.
Figures


Similar articles
-
Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers.J Natl Cancer Inst. 2007 Nov 7;99(21):1623-33. doi: 10.1093/jnci/djm198. Epub 2007 Oct 30. J Natl Cancer Inst. 2007. PMID: 17971529 Free PMC article.
-
Characterization of a full-length infectious cDNA clone and a GFP reporter derivative of the oncolytic picornavirus SVV-001.J Gen Virol. 2012 Dec;93(Pt 12):2606-2613. doi: 10.1099/vir.0.046011-0. Epub 2012 Sep 12. J Gen Virol. 2012. PMID: 22971818
-
Oncolytic Seneca Valley Virus: past perspectives and future directions.Oncolytic Virother. 2016 Sep 6;5:81-9. doi: 10.2147/OV.S96915. eCollection 2016. Oncolytic Virother. 2016. PMID: 27660749 Free PMC article. Review.
-
A Structure-Guided Genetic Modification Strategy: Developing Seneca Valley Virus Therapy against Nonsensitive Nonsmall Cell Lung Carcinoma.J Virol. 2023 May 31;97(5):e0045923. doi: 10.1128/jvi.00459-23. Epub 2023 Apr 25. J Virol. 2023. PMID: 37097154 Free PMC article.
-
Evolving role of seneca valley virus and its biomarker TEM8/ANTXR1 in cancer therapeutics.Front Mol Biosci. 2022 Aug 26;9:930207. doi: 10.3389/fmolb.2022.930207. eCollection 2022. Front Mol Biosci. 2022. PMID: 36090051 Free PMC article. Review.
Cited by
-
Immune checkpoint inhibitors for PD-1/PD-L1 axis in combination with other immunotherapies and targeted therapies for non-small cell lung cancer.Front Oncol. 2022 Aug 17;12:948405. doi: 10.3389/fonc.2022.948405. eCollection 2022. Front Oncol. 2022. PMID: 36059606 Free PMC article. Review.
-
Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients With Advanced Solid Cancers.Mol Ther. 2016 Aug;24(8):1492-501. doi: 10.1038/mt.2016.101. Epub 2016 May 16. Mol Ther. 2016. PMID: 27203445 Free PMC article. Clinical Trial.
-
TEM8 in Oncogenesis: Protein Biology, Pre-Clinical Agents, and Clinical Rationale.Cells. 2023 Nov 14;12(22):2623. doi: 10.3390/cells12222623. Cells. 2023. PMID: 37998358 Free PMC article. Review.
-
Developing Picornaviruses for Cancer Therapy.Cancers (Basel). 2019 May 16;11(5):685. doi: 10.3390/cancers11050685. Cancers (Basel). 2019. PMID: 31100962 Free PMC article. Review.
-
Development and Evaluation of a Competitive Enzyme-Linked Immunosorbent Assay Based on Swine Monoclonal Antibodies for Detecting Neutralizing Antibodies against Senecavirus A.Microbiol Spectr. 2023 Jun 15;11(3):e0459922. doi: 10.1128/spectrum.04599-22. Epub 2023 Apr 10. Microbiol Spectr. 2023. PMID: 37036366 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Murray N, Turrisi AT., III A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. - PubMed
-
- Hales LM, Knowles NJ, Reddy PS, Xu L, Hay C, Hallenbeck PL. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. J Gen Virol. 2008;89:1265–1275. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

