Interleukin-1 in the pathogenesis and treatment of inflammatory diseases
- PMID: 21304099
- PMCID: PMC3083294
- DOI: 10.1182/blood-2010-07-273417
Interleukin-1 in the pathogenesis and treatment of inflammatory diseases
Abstract
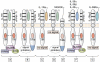
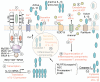
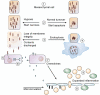
More than any other cytokine family, the IL-1 family of ligands and receptors is primarily associated with acute and chronic inflammation. The cytosolic segment of each IL-1 receptor family member contains the Toll-IL-1-receptor domain. This domain is also present in each Toll-like receptor, the receptors that respond to microbial products and viruses. Since Toll-IL-1-receptor domains are functional for both receptor families, responses to the IL-1 family are fundamental to innate immunity. Of the 11 members of the IL-1 family, IL-1β has emerged as a therapeutic target for an expanding number of systemic and local inflammatory conditions called autoinflammatory diseases. For these, neutralization of IL-1β results in a rapid and sustained reduction in disease severity. Treatment for autoimmune diseases often includes immunosuppressive drugs whereas neutralization of IL-1β is mostly anti-inflammatory. Although some autoinflammatory diseases are due to gain-of-function mutations for caspase-1 activity, common diseases such as gout, type 2 diabetes, heart failure, recurrent pericarditis, rheumatoid arthritis, and smoldering myeloma also are responsive to IL-1β neutralization. This review summarizes acute and chronic inflammatory diseases that are treated by reducing IL-1β activity and proposes that disease severity is affected by the anti-inflammatory members of the IL-1 family of ligands and receptors.
Figures

References
-
- Dinarello CA. Biological basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. - PubMed
-
- Opal SM, Fisher CJJ, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med. 1997;25(7):1115–1124. - PubMed
-
- Eichacker PQ, Parent C, Kalil A, et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166(9):1197–1205. - PubMed
-
- Simon A, van der Meer JW. Pathogenesis of familial periodic fever syndromes or hereditary autoinflammatory syndromes. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R86–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

