Aurora B kinase inhibitor AZD1152: determinants of action and ability to enhance chemotherapeutics effectiveness in pancreatic and colon cancer
- PMID: 21304529
- PMCID: PMC3048212
- DOI: 10.1038/bjc.2011.21
Aurora B kinase inhibitor AZD1152: determinants of action and ability to enhance chemotherapeutics effectiveness in pancreatic and colon cancer
Abstract
Background: AZD1152, the prodrug for AZD1152-hydroxyquinazoline pyrazol anilide (HQPA), is a selective inhibitor of Aurora B kinase activity. Preclinical evaluation of AZD1152 has been reported in several human cancer models. The potentiality of this compound in combination therapy warrants further investigation in solid tumours.
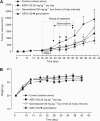
Experimental design: This study explored the effects of AZD1152-HQPA in colon and pancreatic tumour cells. The antitumour properties of AZD1152, either as single agent or in combination with chemotherapeutics, were evaluated in each study model. The efficacy and the toxicity of AZD1152 alone and in combination with gemcitabine were validated in pancreatic tumour xenograft model.
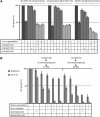
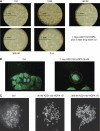
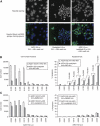
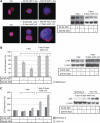
Results: AZD1152-HQPA treatment resulted in a dramatic increase of chromosome number, modification of cell cycle and induction of apoptosis. The most effective combination was that with chemotherapeutics given soon after AZD1152 in both tumour cell types. The effectiveness of the sequential schedule of AZD1152 with gemcitabine was confirmed in nude mice bearing MiaPaCa-2 tumours, showing inhibition of tumour volumes and delaying of tumour growth after the interruption of the treatments. Here we show that AZD1152-HQPA enhances oxaliplatin and gemcitabine effectiveness in colon and pancreatic cancer, respectively. First, we provide advances into administration schedules and dosing regimens for the combination treatment in in vivo pancreatic tumour.
Figures

References
-
- Aihara A, Tanaka S, Yasen M, Matsumura S, Mitsunori Y, Murakata A, Noguchi N, Kudo A, Nakamura N, Ito K, Arii S (2009) The selective Aurora B kinase inhibitor AZD1152 as a novel treatment for hepatocellular carcinoma. J Hepatol 52: 63–71 - PubMed
-
- Azzariti A, Colabufo NA, Berardi F, Porcelli L, Niso M, Simone GM, Perrone R, Paradiso A (2006a) Cyclohexylpiperazine derivative PB28, a sigma2 agonist and sigma1 antagonist receptor, inhibits cell growth, modulates P-glycoprotein, and synergizes with anthracyclines in breast cancer. Mol Cancer Ther 5: 1807–1816 - PubMed
-
- Azzariti A, Porcelli L, Gatti G, Nicolin A, Paradiso A (2008) Synergic antiproliferative and antiangiogenic effects of EGFR and mTor inhibitors on pancreatic cancer cells. Biochem Pharmacol 75: 1035–1044 - PubMed
-
- Azzariti A, Porcelli L, Xu JM, Simone GM, Paradiso A (2006b) Prolonged exposure of colon cancer cells to the epidermal growth factor receptor inhibitor gefitinib (Iressa(TM)) and to the antiangiogenic agent ZD6474: cytotoxic and biomolecular effects. World J Gastroenterol 12: 5140–5147 - PMC - PubMed
-
- Azzariti A, Xu JM, Porcelli L, Paradiso A (2004) The schedule-dependent enhanced cytotoxic activity of 7-ethyl-10-hydroxy-camptothecin (SN-38) in combination with Gefitinib (Iressa, ZD1839). Biochem Pharmacol 68: 135–144 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

