Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle
- PMID: 21304812
- PMCID: PMC3033412
- DOI: 10.1371/journal.pone.0016816
Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle
Abstract
Background: Therapy for neural lesions or degenerative diseases relies mainly on finding transplantable active precursor cells. Identifying them in peripheral tissues accessible for biopsy, outside the central nervous system, would circumvent the serious immunological and ethical concerns impeding cell therapy.
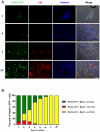
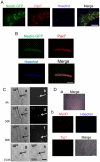
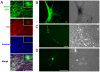
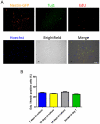
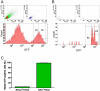
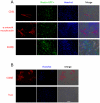
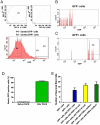
Methodology/principal findings: In this study, we isolated neural progenitor cells in cultured adult skeletal muscle from transgenic mice in which nestin regulatory elements control GFP expression. These cells also expressed the early neural marker Tuj1 and light and heavy neurofilament but not S100β, indicating that they express typical neural but not Schwann cell markers. GFP+/Tuj1+ cells were also negative for the endothelial and pericyte markers CD31 and α-smooth muscle actin, respectively. We established their a) functional response to glutamate in patch-clamp recordings; b) interstitial mesenchymal origin; c) replicative capacity; and d) the environment necessary for their survival after fluorescence-activated cell sorting.
Conclusions/significance: We propose that the decline in nestin-GFP expression in muscle progenitor cells and its persistence in neural precursor cells in muscle cultures provide an invaluable tool for isolating a population of predifferentiated neural cells with therapeutic potential.
Conflict of interest statement
Figures


References
-
- Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. - PubMed
-
- Salgado AJ, Oliveira JT, Pedro AJ, Reis RL. Adult stem cells in bone and cartilage tissue engineering. Curr Stem Cell Res Ther. 2006;1:345–364. - PubMed
-
- Zipori D. The stem state: mesenchymal plasticity as a paradigm. Curr Stem Cell Res Ther. 2006;1:95–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

