Interferon gamma-dependent intestinal pathology contributes to the lethality in bacterial superantigen-induced toxic shock syndrome
- PMID: 21304813
- PMCID: PMC3033413
- DOI: 10.1371/journal.pone.0016764
Interferon gamma-dependent intestinal pathology contributes to the lethality in bacterial superantigen-induced toxic shock syndrome
Abstract
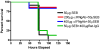
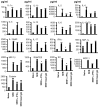
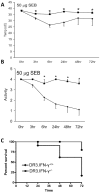
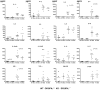
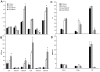
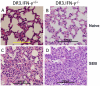
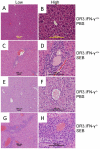
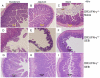
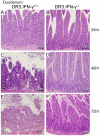
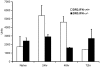
Toxic shock syndrome (TSS) caused by the superantigen exotoxins of Staphylococcus aureus and Streptococcus pyogenes is characterized by robust T cell activation, profound elevation in systemic levels of multiple cytokines, including interferon-γ (IFN-γ), followed by multiple organ dysfunction and often death. As IFN-γ possesses pro- as well as anti-inflammatory properties, we delineated its role in the pathogenesis of TSS. Antibody-mediated in vivo neutralization of IFN-γ or targeted disruption of IFN-γ gene conferred significant protection from lethal TSS in HLA-DR3 transgenic mice. Following systemic high dose SEB challenge, whereas the HLA-DR3.IFN-γ(+/+) mice became sick and succumbed to TSS, HLA-DR3.IFN-γ(-/-) mice appeared healthy and were significantly protected from SEB-induced lethality. SEB-induced systemic cytokine storm was significantly blunted in HLA-DR3.IFN-γ(-/-) transgenic mice. Serum concentrations of several cytokines (IL-4, IL-10, IL-12p40 and IL-17) and chemokines (KC, rantes, eotaxin and MCP-1) were significantly lower in HLA-DR3.IFN-γ(-/-) transgenic mice. However, SEB-induced T cell expansion in the spleens was unaffected and expansion of SEB-reactive TCR Vβ8(+) CD4(+) and CD8(+) T cells was even more pronounced in HLA-DR3.IFN-γ(-/-) transgenic mice when compared to HLA-DR3.IFN-γ(+/+) mice. A systematic histopathological examination of several vital organs revealed that both HLA-DR3.IFN-γ(+/+) and HLA-DR3.IFN-γ(-/-) transgenic mice displayed comparable severe inflammatory changes in lungs, and liver during TSS. Remarkably, whereas the small intestines from HLA-DR3.IFN-γ(+/+) transgenic mice displayed significant pathological changes during TSS, the architecture of small intestines in HLA-DR3.IFN-γ(-/-) transgenic mice was preserved. In concordance with these histopathological changes, the gut permeability to macromolecules was dramatically increased in HLA-DR3.IFN-γ(+/+) but not HLA-DR3.IFN-γ(-/-) mice during TSS. Overall, IFN-γ seemed to play a lethal role in the immunopathogenesis of TSS by inflicting fatal small bowel pathology. Our study thus identifies the important role for IFN-γ in TSS.
Conflict of interest statement
Figures
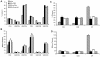

Similar articles
-
Systemic inflammatory response elicited by superantigen destabilizes T regulatory cells, rendering them ineffective during toxic shock syndrome.J Immunol. 2014 Sep 15;193(6):2919-30. doi: 10.4049/jimmunol.1400980. Epub 2014 Aug 4. J Immunol. 2014. PMID: 25092888 Free PMC article.
-
Chimeric anti-staphylococcal enterotoxin B antibodies and lovastatin act synergistically to provide in vivo protection against lethal doses of SEB.PLoS One. 2011;6(11):e27203. doi: 10.1371/journal.pone.0027203. Epub 2011 Nov 15. PLoS One. 2011. PMID: 22102880 Free PMC article.
-
"Small" Intestinal Immunopathology Plays a "Big" Role in Lethal Cytokine Release Syndrome, and Its Modulation by Interferon-γ, IL-17A, and a Janus Kinase Inhibitor.Front Immunol. 2020 Jun 26;11:1311. doi: 10.3389/fimmu.2020.01311. eCollection 2020. Front Immunol. 2020. PMID: 32676080 Free PMC article.
-
Staphylococcal Superantigens: Pyrogenic Toxins Induce Toxic Shock.Toxins (Basel). 2019 Mar 23;11(3):178. doi: 10.3390/toxins11030178. Toxins (Basel). 2019. PMID: 30909619 Free PMC article. Review.
-
Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products.Pathophysiology. 2024 Jan 9;31(1):18-31. doi: 10.3390/pathophysiology31010002. Pathophysiology. 2024. PMID: 38251046 Free PMC article. Review.
Cited by
-
Systemic inflammatory response elicited by superantigen destabilizes T regulatory cells, rendering them ineffective during toxic shock syndrome.J Immunol. 2014 Sep 15;193(6):2919-30. doi: 10.4049/jimmunol.1400980. Epub 2014 Aug 4. J Immunol. 2014. PMID: 25092888 Free PMC article.
-
The impact of Staphylococcus aureus-associated molecular patterns on staphylococcal superantigen-induced toxic shock syndrome and pneumonia.Mediators Inflamm. 2014;2014:468285. doi: 10.1155/2014/468285. Epub 2014 Jun 12. Mediators Inflamm. 2014. PMID: 25024509 Free PMC article.
-
Rapid and Rigorous IL-17A Production by a Distinct Subpopulation of Effector Memory T Lymphocytes Constitutes a Novel Mechanism of Toxic Shock Syndrome Immunopathology.J Immunol. 2017 Apr 1;198(7):2805-2818. doi: 10.4049/jimmunol.1601366. Epub 2017 Feb 20. J Immunol. 2017. PMID: 28219889 Free PMC article.
-
Chimeric anti-staphylococcal enterotoxin B antibodies and lovastatin act synergistically to provide in vivo protection against lethal doses of SEB.PLoS One. 2011;6(11):e27203. doi: 10.1371/journal.pone.0027203. Epub 2011 Nov 15. PLoS One. 2011. PMID: 22102880 Free PMC article.
-
Induction of intestinal pro-inflammatory immune responses by lipoteichoic acid.J Inflamm (Lond). 2012 Mar 16;9:7. doi: 10.1186/1476-9255-9-7. J Inflamm (Lond). 2012. PMID: 22423982 Free PMC article.
References
-
- Yok-Al Que, Moreill P. Staphylococcus aureus (Including Staphylococcal Toxic Shock). In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases 9ed. Philadelphia: Churchill Livingstone, Elsevier; 2009.
-
- Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9:281–290. - PubMed
-
- Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii Infection: Epidemiology, Clinical Findings, and Current Perspectives on Diagnosis and Treatment. Clinical Infectious Diseases. 2006;43:1436–1446. - PubMed
-
- Murray RJ. Recognition and management of Staphylococcus aureus toxin-mediated disease. Internal Medicine Journal. 2005;35:S106–S119. - PubMed
-
- Todd JK. Staphylococcal Toxin Syndromes. Annual Review of Medicine. 1985;36:337–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

