Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation
- PMID: 21304882
- PMCID: PMC3033375
- DOI: 10.1371/journal.ppat.1001271
Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation
Abstract
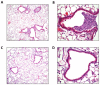
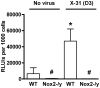
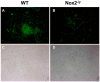
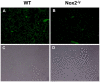
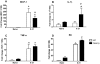
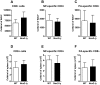
Influenza A virus pandemics and emerging anti-viral resistance highlight the urgent need for novel generic pharmacological strategies that reduce both viral replication and lung inflammation. We investigated whether the primary enzymatic source of inflammatory cell ROS (reactive oxygen species), Nox2-containing NADPH oxidase, is a novel pharmacological target against the lung inflammation caused by influenza A viruses. Male WT (C57BL/6) and Nox2(-/y) mice were infected intranasally with low pathogenicity (X-31, H3N2) or higher pathogenicity (PR8, H1N1) influenza A virus. Viral titer, airways inflammation, superoxide and peroxynitrite production, lung histopathology, pro-inflammatory (MCP-1) and antiviral (IL-1β) cytokines/chemokines, CD8(+) T cell effector function and alveolar epithelial cell apoptosis were assessed. Infection of Nox2(-/y) mice with X-31 virus resulted in a significant reduction in viral titers, BALF macrophages, peri-bronchial inflammation, BALF inflammatory cell superoxide and lung tissue peroxynitrite production, MCP-1 levels and alveolar epithelial cell apoptosis when compared to WT control mice. Lung levels of IL-1β were ∼3-fold higher in Nox2(-/y) mice. The numbers of influenza-specific CD8+D(b)NP(366)+ and D(b)PA(224)+ T cells in the BALF and spleen were comparable in WT and Nox2(-/y) mice. In vivo administration of the Nox2 inhibitor apocynin significantly suppressed viral titer, airways inflammation and inflammatory cell superoxide production following infection with X-31 or PR8. In conclusion, these findings indicate that Nox2 inhibitors have therapeutic potential for control of lung inflammation and damage in an influenza strain-independent manner.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

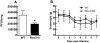

References
-
- La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. - PubMed
-
- Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. - PubMed
-
- Oda T, Akaike T, Hamamoto T, Suzuki F, Hirano T, et al. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989;244:974–976. - PubMed
-
- Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

