Nucleosomes containing methylated DNA stabilize DNA methyltransferases 3A/3B and ensure faithful epigenetic inheritance
- PMID: 21304883
- PMCID: PMC3033376
- DOI: 10.1371/journal.pgen.1001286
Nucleosomes containing methylated DNA stabilize DNA methyltransferases 3A/3B and ensure faithful epigenetic inheritance
Abstract
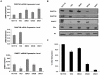
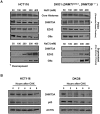
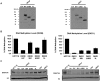
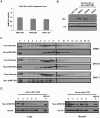
How epigenetic information is propagated during somatic cell divisions is still unclear but is absolutely critical for preserving gene expression patterns and cellular identity. Here we show an unanticipated mechanism for inheritance of DNA methylation patterns where the epigenetic mark not only recruits the catalyzing enzyme but also regulates the protein level, i.e. the enzymatic product (5-methylcytosine) determines the level of the methylase, thus forming a novel homeostatic inheritance system. Nucleosomes containing methylated DNA stabilize de novo DNA methyltransferases, DNMT3A/3B, allowing little free DNMT3A/3B enzymes to exist in the nucleus. Stabilization of DNMT3A/3B on nucleosomes in methylated regions further promotes propagation of DNA methylation. However, reduction of cellular DNA methylation levels creating more potential CpG substrates counter-intuitively results in a dramatic decrease of DNMT3A/3B proteins due to diminished nucleosome binding and subsequent degradation of the unstable free proteins. These data show an unexpected self-regulatory inheritance mechanism that not only ensures somatic propagation of methylated states by DNMT1 and DNMT3A/3B enzymes but also prevents aberrant de novo methylation by causing degradation of free DNMT3A/3B enzymes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. - PubMed
-
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. - PubMed
-
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

