In situ photodegradation of incorporated polyanion does not alter prion infectivity
- PMID: 21304885
- PMCID: PMC3033378
- DOI: 10.1371/journal.ppat.1002001
In situ photodegradation of incorporated polyanion does not alter prion infectivity
Abstract
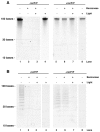
Single-stranded polyanions ≥40 bases in length facilitate the formation of hamster scrapie prions in vitro, and polyanions co-localize with PrP(Sc) aggregates in vivo. To test the hypothesis that intact polyanionic molecules might serve as a structural backbone essential for maintaining the infectious conformation(s) of PrP(Sc), we produced synthetic prions using a photocleavable, 100-base oligonucleotide (PC-oligo). In serial Protein Misfolding Cyclic Amplification (sPMCA) reactions using purified PrP(C) substrate, PC-oligo was incorporated into physical complexes with PrP(Sc) molecules that were resistant to benzonase digestion. Exposure of these nuclease-resistant prion complexes to long wave ultraviolet light (315 nm) induced degradation of PC-oligo into 5 base fragments. Light-induced photolysis of incorporated PC-oligo did not alter the infectivity of in vitro-generated prions, as determined by bioassay in hamsters and brain homogenate sPMCA assays. Neuropathological analysis also revealed no significant differences in the neurotropism of prions containing intact versus degraded PC-oligo. These results show that polyanions >5 bases in length are not required for maintaining the infectious properties of in vitro-generated scrapie prions, and indicate that such properties are maintained either by short polyanion remnants, other co-purified cofactors, or by PrP(Sc) molecules alone.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


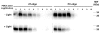

 -). The mean values (n = 5–8 animals/group) are shown ± S.E.M. FC: Frontal cortex. PC: Parietal cortex. H: Hippocampus. C: Cerebellum. BS: Brain stem.
-). The mean values (n = 5–8 animals/group) are shown ± S.E.M. FC: Frontal cortex. PC: Parietal cortex. H: Hippocampus. C: Cerebellum. BS: Brain stem.References
-
- Snow AD, Wight TN, Nochlin D, Koike Y, Kimata K, et al. Immunolocalization of heparan sulfate proteoglycans to the prion protein amyloid plaques of Gerstmann-Straussler syndrome, Creutzfeldt- Jakob disease and scrapie. Lab Invest. 1990;63:601–611. - PubMed
-
- Basler K, Oesch B, Scott M, Westaway D, Walchli M, et al. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986;46:417–428. - PubMed
-
- Castilla J, Saa P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

