Pathogenic VCP/TER94 alleles are dominant actives and contribute to neurodegeneration by altering cellular ATP level in a Drosophila IBMPFD model
- PMID: 21304887
- PMCID: PMC3033380
- DOI: 10.1371/journal.pgen.1001288
Pathogenic VCP/TER94 alleles are dominant actives and contribute to neurodegeneration by altering cellular ATP level in a Drosophila IBMPFD model
Abstract
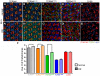
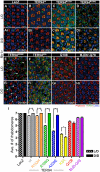
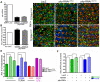
Inclusion body myopathy with Paget's disease of bone and frontotemporal dementia (IBMPFD) is caused by mutations in Valosin-containing protein (VCP), a hexameric AAA ATPase that participates in a variety of cellular processes such as protein degradation, organelle biogenesis, and cell-cycle regulation. To understand how VCP mutations cause IBMPFD, we have established a Drosophila model by overexpressing TER94 (the sole Drosophila VCP ortholog) carrying mutations analogous to those implicated in IBMPFD. Expression of these TER94 mutants in muscle and nervous systems causes tissue degeneration, recapitulating the pathogenic phenotypes in IBMPFD patients. TER94-induced neurodegenerative defects are enhanced by elevated expression of wild-type TER94, suggesting that the pathogenic alleles are dominant active mutations. This conclusion is further supported by the observation that TER94-induced neurodegenerative defects require the formation of hexamer complex, a prerequisite for a functional AAA ATPase. Surprisingly, while disruptions of the ubiquitin-proteasome system (UPS) and the ER-associated degradation (ERAD) have been implicated as causes for VCP-induced tissue degeneration, these processes are not significantly affected in our fly model. Instead, the neurodegenerative defect of TER94 mutants seems sensitive to the level of cellular ATP. We show that increasing cellular ATP by independent mechanisms could suppress the phenotypes of TER94 mutants. Conversely, decreasing cellular ATP would enhance the TER94 mutant phenotypes. Taken together, our analyses have defined the nature of IBMPFD-causing VCP mutations and made an unexpected link between cellular ATP level and IBMPFD pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
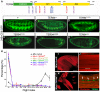
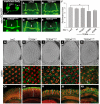
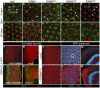



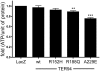
References
-
- Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. - PubMed
-
- Dai RM, Li CC. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol. 2001;3:740–744. - PubMed
-
- Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, et al. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol. 2002;4:134–139. - PubMed
-
- Latterich M, Fröhlich KU, Schekman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995;82:885–893. - PubMed
-
- Rabouille C, Levine TP, Peters JM, Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995;82:905–914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

