A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus
- PMID: 21304910
- PMCID: PMC3033404
- DOI: 10.1371/journal.pone.0014646
A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus
Abstract
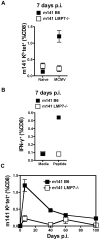
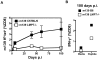
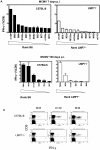
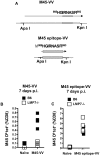
Murine cytomegalovirus (MCMV) is an important animal model of human cytomegalovirus (HCMV), a β-Herpesvirus that infects the majority of the world's population and causes disease in neonates and immunocompromised adults. CD8(+) T cells are a major part of the immune response to MCMV and HCMV. Processing of peptides for presentation to CD8(+) T cells may be critically dependent on the immunoproteasome, expression of which is affected by MCMV. However, the overall importance of the immunoproteasome in the generation of immunodominant peptides from MCMV is not known. We therefore examined the role of the immunoproteasome in stimulation of CD8(+) T cell responses to MCMV - both conventional memory responses and those undergoing long-term expansion or "inflation". We infected LMP7(-/-) and C57BL/6 mice with MCMV or with newly-generated recombinant vaccinia viruses (rVVs) encoding the immunodominant MCMV protein M45 in either full-length or epitope-only minigene form. We analysed CD8(+) T cell responses using intracellular cytokine stain (ICS) and MHC Class I tetramer staining for a panel of MCMV-derived epitopes. We showed a critical role for immunoproteasome in MCMV affecting all epitopes studied. Interestingly we found that memory "inflating" epitopes demonstrate reduced immunoproteasome dependence compared to non-inflating epitopes. M45-specific responses induced by rVVs remain immunoproteasome-dependent. These results help to define a critical restriction point for CD8(+) T cell epitopes in natural cytomegalovirus (CMV) infection and potentially in vaccine strategies against this and other viruses.
Conflict of interest statement
Figures


Similar articles
-
Viral interference with antigen presentation does not alter acute or chronic CD8 T cell immunodominance in murine cytomegalovirus infection.J Immunol. 2007 Jun 1;178(11):7235-41. doi: 10.4049/jimmunol.178.11.7235. J Immunol. 2007. PMID: 17513772
-
The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo.J Immunol. 2002 Jul 1;169(1):359-65. doi: 10.4049/jimmunol.169.1.359. J Immunol. 2002. PMID: 12077265
-
Batf3 transcription factor-dependent DC subsets in murine CMV infection: differential impact on T-cell priming and memory inflation.Eur J Immunol. 2011 Sep;41(9):2612-8. doi: 10.1002/eji.201041075. Epub 2011 Aug 4. Eur J Immunol. 2011. PMID: 21604258
-
Caspase-8-dependent control of NK- and T cell responses during cytomegalovirus infection.Med Microbiol Immunol. 2019 Aug;208(3-4):555-571. doi: 10.1007/s00430-019-00616-7. Epub 2019 May 16. Med Microbiol Immunol. 2019. PMID: 31098689 Review.
-
Immune and non-immune functions of the immunoproteasome.Front Biosci (Landmark Ed). 2012 Jan 1;17(5):1904-16. doi: 10.2741/4027. Front Biosci (Landmark Ed). 2012. PMID: 22201844 Review.
Cited by
-
Overall avidity declines in TCR repertoires during latent CMV but not EBV infection.Front Immunol. 2023 Nov 20;14:1293090. doi: 10.3389/fimmu.2023.1293090. eCollection 2023. Front Immunol. 2023. PMID: 38053994 Free PMC article.
-
Memory Inflation Drives Tissue-Resident Memory CD8+ T Cell Maintenance in the Lung After Intranasal Vaccination With Murine Cytomegalovirus.Front Immunol. 2018 Aug 14;9:1861. doi: 10.3389/fimmu.2018.01861. eCollection 2018. Front Immunol. 2018. PMID: 30154789 Free PMC article.
-
Immunoproteasome Subunits Are Required for CD8+ T Cell Function and Host Resistance to Brucella abortus Infection in Mice.Infect Immun. 2018 Feb 20;86(3):e00615-17. doi: 10.1128/IAI.00615-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29263103 Free PMC article.
-
Cytomegalovirus memory inflation and immune protection.Med Microbiol Immunol. 2019 Aug;208(3-4):339-347. doi: 10.1007/s00430-019-00607-8. Epub 2019 Apr 10. Med Microbiol Immunol. 2019. PMID: 30972476 Review.
-
Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome.Proc Natl Acad Sci U S A. 2011 Sep 6;108(36):14914-9. doi: 10.1073/pnas.1106015108. Epub 2011 Aug 18. Proc Natl Acad Sci U S A. 2011. PMID: 21852578 Free PMC article.
References
-
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. - PubMed
-
- Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. - PubMed
-
- Podlech J, Holtappels R, Wirtz N, Steffens HP, Reddehase MJ. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol. 1998;79(Pt 9):2099–2104. - PubMed
-
- Moss P, Khan N. CD8(+) T-cell immunity to cytomegalovirus. Hum Immunol. 2004;65:456–464. - PubMed
-
- Reddehase MJ, Keil GM, Koszinowski UH. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984;14:56–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

