Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial
- PMID: 21304923
- PMCID: PMC3032550
- DOI: 10.1371/journal.pmed.1000407
Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial
Abstract
Background: Previous studies have shown that in areas of seasonal malaria transmission, intermittent preventive treatment of malaria in children (IPTc), targeting the transmission season, reduces the incidence of clinical malaria. However, these studies were conducted in communities with low coverage with insecticide-treated nets (ITNs). Whether IPTc provides additional protection to children sleeping under an ITN has not been established.
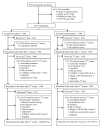
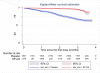
Methods and findings: To assess whether IPTc provides additional protection to children sleeping under an ITN, we conducted a randomised, double-blind, placebo-controlled trial of IPTc with sulphadoxine pyrimethamine (SP) plus amodiaquine (AQ) in three localities in Kati, Mali. After screening, eligible children aged 3-59 mo were given a long-lasting insecticide-treated net (LLIN) and randomised to receive three rounds of active drugs or placebos. Treatments were administered under observation at monthly intervals during the high malaria transmission season in August, September, and October 2008. Adverse events were monitored immediately after the administration of each course of IPTc and throughout the follow-up period. The primary endpoint was clinical episodes of malaria recorded through passive surveillance by study clinicians available at all times during the follow-up. Cross-sectional surveys were conducted in 150 randomly selected children weekly and in all children at the end of the malaria transmission season to assess usage of ITNs and the impact of IPTc on the prevalence of malaria, anaemia, and malnutrition. Cox regression was used to compare incidence rates between intervention and control arms. The effects of IPTc on the prevalence of malaria infection and anaemia were estimated using logistic regression. 3,065 children were screened and 3,017 (1,508 in the control and 1,509 in the intervention arm) were enrolled in the study. 1,485 children (98.5%) in the control arm and 1,481 (98.1%) in the intervention arm completed follow-up. During the intervention period, the proportion of children reported to have slept under an ITN was 99.7% in the control and 99.3% in intervention arm (p = 0.45). A total of 672 episodes of clinical malaria defined as fever or a history of fever and the presence of at least 5,000 asexual forms of Plasmodium falciparum per microlitre (incidence rate of 1.90; 95% confidence interval [CI] 1.76-2.05 episodes per person year) were observed in the control arm versus 126 (incidence rate of 0.34; 95% CI 0.29-0.41 episodes per person year) in the intervention arm, indicating a protective effect (PE) of 82% (95% CI 78%-85%) (p<0.001) on the primary endpoint. There were 15 episodes of severe malaria in children in the control arm compared to two in children in the intervention group giving a PE of 87% (95% CI 42%-99%) (p = 0.001). IPTc reduced the prevalence of malaria infection by 85% (95% CI 73%-92%) (p<0.001) during the intervention period and by 46% (95% CI 31%-68%) (p<0.001) at the end of the intervention period. The prevalence of moderate anaemia (haemoglobin [Hb] <8 g/dl) was reduced by 47% (95% CI 15%-67%) (p<0.007) at the end of intervention period. The frequencies of adverse events were similar between the two arms. There was no drug-related serious adverse event.
Conclusions: IPTc given during the malaria transmission season provided substantial protection against clinical episodes of malaria, malaria infection, and anaemia in children using an LLIN. SP+AQ was safe and well tolerated. These findings indicate that IPTc could make a valuable contribution to malaria control in areas of seasonal malaria transmission alongside other interventions.
Trial registration: ClinicalTrials.gov NCT00738946. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- United Nations. 2008. World population prospects. The 2008 revision volume ii: sex and age distribution of the world population. Available: http://esa.un.org/unpd/wpp2008/peps_documents.htm Accessed 21 November 2010.
-
- WHO. 2008. World malaria report, 2008. Available: http://www.who.int/malaria/wmr2008/malaria2008.pdf Accessed 10 May 2010.
-
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004:CD000363. - PubMed
-
- Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised,placebo-controlled trials. Lancet. 2009;374:1533–1542. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical