Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against varicella-zoster virus
- PMID: 21304940
- PMCID: PMC3033373
- DOI: 10.1371/journal.ppat.1001266
Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against varicella-zoster virus
Abstract
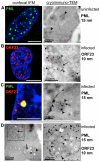
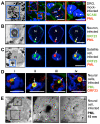
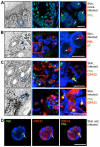
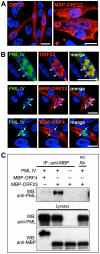
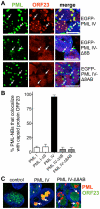
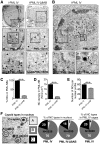
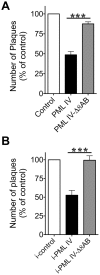
The herpesviruses, like most other DNA viruses, replicate in the host cell nucleus. Subnuclear domains known as promyelocytic leukemia protein nuclear bodies (PML-NBs), or ND10 bodies, have been implicated in restricting early herpesviral gene expression. These viruses have evolved countermeasures to disperse PML-NBs, as shown in cells infected in vitro, but information about the fate of PML-NBs and their functions in herpesvirus infected cells in vivo is limited. Varicella-zoster virus (VZV) is an alphaherpesvirus with tropism for skin, lymphocytes and sensory ganglia, where it establishes latency. Here, we identify large PML-NBs that sequester newly assembled nucleocapsids (NC) in neurons and satellite cells of human dorsal root ganglia (DRG) and skin cells infected with VZV in vivo. Quantitative immuno-electron microscopy revealed that these distinctive nuclear bodies consisted of PML fibers forming spherical cages that enclosed mature and immature VZV NCs. Of six PML isoforms, only PML IV promoted the sequestration of NCs. PML IV significantly inhibited viral infection and interacted with the ORF23 capsid surface protein, which was identified as a target for PML-mediated NC sequestration. The unique PML IV C-terminal domain was required for both capsid entrapment and antiviral activity. Similar large PML-NBs, termed clastosomes, sequester aberrant polyglutamine (polyQ) proteins, such as Huntingtin (Htt), in several neurodegenerative disorders. We found that PML IV cages co-sequester HttQ72 and ORF23 protein in VZV infected cells. Our data show that PML cages contribute to the intrinsic antiviral defense by sensing and entrapping VZV nucleocapsids, thereby preventing their nuclear egress and inhibiting formation of infectious virus particles. The efficient sequestration of virion capsids in PML cages appears to be the outcome of a basic cytoprotective function of this distinctive category of PML-NBs in sensing and safely containing nuclear aggregates of aberrant proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Zhong S, Muller S, Ronchetti S, Freemont PS, Dejean A, et al. Role of SUMO-1-modified PML in nuclear body formation. Blood. 2000;95:2748–2752. - PubMed
-
- Eskiw CH, Dellaire G, Mymryk JS, Bazett-Jones DP. Size, position and dynamic behavior of PML nuclear bodies following cell stress as a paradigm for supramolecular trafficking and assembly. J Cell Sci. 2003;116:4455–4466. - PubMed
-
- Muratani M, Gerlich D, Janicki SM, Gebhard M, Eils R, et al. Metabolic-energy-dependent movement of PML bodies within the mammalian cell nucleus. Nat Cell Biol. 2002;4:106–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

