Immunogenicity and cross-reactivity of 2009-2010 inactivated seasonal influenza vaccine in US adults and elderly
- PMID: 21304946
- PMCID: PMC3031605
- DOI: 10.1371/journal.pone.0016650
Immunogenicity and cross-reactivity of 2009-2010 inactivated seasonal influenza vaccine in US adults and elderly
Abstract
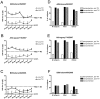
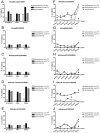
The campaign of 2009-2010 Northern Hemisphere seasonal vaccination was concurrent with the 2009 H1N1 pandemic. Using a hemagglutination inhibition (HAI) assay, we evaluated the immunogenicity and cross-reactivity of 2009-2010 inactivated trivalent influenza vaccine (TIV) in US adult and elderly populations. Vaccination of TIV resulted in a robust boost on the antibody response of all subjects to seasonal A/Brisbane/59/2007 (H1N1) and A/Uruguay/716/2007 (H3N2) with over 70% of recipients reaching a seroprotective titer of 40. B/Brisbane/60/2008 was the least immunogenic among the three seasonal vaccine strains with <30% of TIV recipients reaching a seroprotective titer of 40. TIV vaccination also induced a moderate boost on the pandemic specific antibody responses. Twenty-four percent of adults and 36% of elderly reached a seroprotective HAI titer of 40 or more against pandemic A/South Carolina/18/2009 (H1N1) after receiving TIV compared to 4% and 7% at the beginning of vaccination, respectively. In addition, 22% of adults and 34% of elderly showed an increase of 4-fold or more in A/South Carolina/18/2009 specific HAI titers after TIV vaccination. The pandemic specific cross-reactive antibodies strongly correlated with the post-vaccination HAI titers against the seasonal H3N2 vaccine strain in all subjects.
Conflict of interest statement
Figures

References
-
- CDC. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58(19):521–524. - PubMed
-
- de Jong JC, Palache AM, Beyer WEP, Rimmelzwaan GF, Boon ACM, et al. Haemagglutination-inhibiting antibodies to influenza virus. Dev Biol (Basel) 2003;115:63–73. - PubMed
-
- Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. - PubMed
-
- Fox JP, Hall CE, Cooney MK, Foy HM. Influenza virus infections in Seattle families, 1975–1979. I. Study design, methods and the occurrence of infections by time and age. Am J Epidemiol. 1982;116:212–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

