Skeletal muscle 11beta-HSD1 controls glucocorticoid-induced proteolysis and expression of E3 ubiquitin ligases atrogin-1 and MuRF-1
- PMID: 21304964
- PMCID: PMC3031623
- DOI: 10.1371/journal.pone.0016674
Skeletal muscle 11beta-HSD1 controls glucocorticoid-induced proteolysis and expression of E3 ubiquitin ligases atrogin-1 and MuRF-1
Abstract
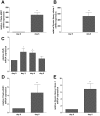
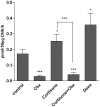
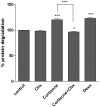
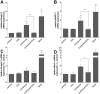
Recent studies demonstrated expression and activity of the intracellular cortisone-cortisol shuttle 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) in skeletal muscle and inhibition of 11beta-HSD1 in muscle cells improved insulin sensitivity. Glucocorticoids induce muscle atrophy via increased expression of the E3 ubiquitin ligases Atrogin-1 (Muscle Atrophy F-box (MAFbx)) and MuRF-1 (Muscle RING-Finger-1). We hypothesized that 11beta-HSD1 controls glucocorticoid-induced expression of atrophy E3 ubiquitin ligases in skeletal muscle. Primary human myoblasts were generated from healthy volunteers. 11beta-HSD1-dependent protein degradation was analyzed by [(3)H]-tyrosine release assay. RT-PCR was used to determine mRNA expression of E3 ubiquitin ligases and 11beta-HSD1 activity was measured by conversion of radioactively labeled [(3)H]-cortisone to [(3)H]-cortisol separated by thin-layer chromatography. We here demonstrate that 11beta-HSD1 is expressed and biologically active in interconverting cortisone to active cortisol in murine skeletal muscle cells (C2C12) as well as in primary human myotubes. 11Beta-HSD1 expression increased during differentiation from myoblasts to mature myotubes (p < 0.01), suggesting a role of 11beta-HSD1 in skeletal muscle growth and differentiation. Treatment with cortisone increased protein degradation by about 20% (p < 0.001), which was paralleled by an elevation of Atrogin-1 and MuRF-1 mRNA expression (p < 0.01, respectively). Notably, pre-treatment with the 11beta-HSD1 inhibitor carbenoxolone (Cbx) completely abolished the effect of cortisone on protein degradation as well as on Atrogin-1 and MuRF-1 expression. In summary, our data suggest that 11beta-HSD1 controls glucocorticoid-induced protein degradation in human and murine skeletal muscle via regulation of the E3 ubiquitin ligases Atrogin-1 and MuRF-1.
Conflict of interest statement
Figures
References
-
- Lindsay RS, Wake DJ, Nair S, Bunt J, Livingstone DE, et al. Subcutaneous adipose 11 beta-hydroxysteroid dehydrogenase type 1 activity and messenger ribonucleic acid levels are associated with adiposity and insulinemia in Pima Indians and Caucasians. J Clin Endocrinol Metab. 2003;88:2738–2744. - PubMed
-
- Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Heintze U, et al. Regulation of 11beta-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes Res. 2004;12:9–17. - PubMed
-
- Mariniello B, Ronconi V, Rilli S, Bernante P, Boscaro M, et al. Adipose tissue 11beta-hydroxysteroid dehydrogenase type 1 expression in obesity and Cushing's syndrome. Eur J Endocrinol. 2006;155:435–441. - PubMed
-
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

