Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase
- PMID: 21304987
- PMCID: PMC3031585
- DOI: 10.1371/journal.pone.0016571
Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase
Abstract
Background: Earlier we have shown that extracellular sphingosine-1-phosphate (S1P) induces migration of human pulmonary artery endothelial cells (HPAECs) through the activation of S1P(1) receptor, PKCε, and PLD2-PKCζ-Rac1 signaling cascade. As endothelial cells generate intracellular S1P, here we have investigated the role of sphingosine kinases (SphKs) and S1P lyase (S1PL), that regulate intracellular S1P accumulation, in HPAEC motility.
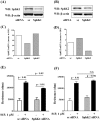
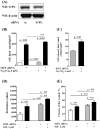
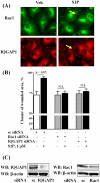
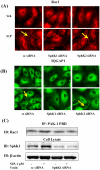
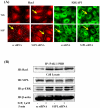
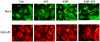
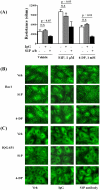
Methodology/principal findings: Inhibition of SphK activity with a SphK inhibitor 2-(p-Hydroxyanilino)-4-(p-Chlorophenyl) Thiazole or down-regulation of Sphk1, but not SphK2, with siRNA decreased S1P(int), and attenuated S1P(ext) or serum-induced motility of HPAECs. On the contrary, inhibition of S1PL with 4-deoxypyridoxine or knockdown of S1PL with siRNA increased S1P(int) and potentiated motility of HPAECs to S1P(ext) or serum. S1P(ext) mediates cell motility through activation of Rac1 and IQGAP1 signal transduction in HPAECs. Silencing of SphK1 by siRNA attenuated Rac1 and IQGAP1 translocation to the cell periphery; however, knockdown of S1PL with siRNA or 4-deoxypyridoxine augmented activated Rac1 and stimulated Rac1 and IQGAP1 translocation to cell periphery. The increased cell motility mediated by down-regulation was S1PL was pertussis toxin sensitive suggesting "inside-out" signaling of intracellularly generated S1P. Although S1P did not accumulate significantly in media under basal or S1PL knockdown conditions, addition of sodium vanadate increased S1P levels in the medium and inside the cells most likely by blocking phosphatases including lipid phosphate phosphatases (LPPs). Furthermore, addition of anti-S1P mAb to the incubation medium blocked S1P(ext) or 4-deoxypyridoxine-dependent endothelial cell motility.
Conclusions/significance: These results suggest S1P(ext) mediated endothelial cell motility is dependent on intracellular S1P production, which is regulated, in part, by SphK1 and S1PL.
Conflict of interest statement
Figures






Similar articles
-
Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways.Am J Physiol Lung Cell Mol Physiol. 2011 Jun;300(6):L840-50. doi: 10.1152/ajplung.00404.2010. Epub 2011 Apr 8. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21478254 Free PMC article.
-
Role of Sphingosine Kinase 1 and S1P Transporter Spns2 in HGF-mediated Lamellipodia Formation in Lung Endothelium.J Biol Chem. 2016 Dec 30;291(53):27187-27203. doi: 10.1074/jbc.M116.758946. Epub 2016 Nov 18. J Biol Chem. 2016. PMID: 27864331 Free PMC article.
-
Sphingolipids in pulmonary fibrosis.Adv Biol Regul. 2015 Jan;57:55-63. doi: 10.1016/j.jbior.2014.09.008. Epub 2014 Oct 13. Adv Biol Regul. 2015. PMID: 25446881 Free PMC article. Review.
-
Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1.J Biol Chem. 2007 May 11;282(19):14165-77. doi: 10.1074/jbc.M701279200. Epub 2007 Mar 22. J Biol Chem. 2007. PMID: 17379599 Free PMC article.
-
Epigenetic regulation of pro-inflammatory cytokine secretion by sphingosine 1-phosphate (S1P) in acute lung injury: Role of S1P lyase.Adv Biol Regul. 2017 Jan;63:156-166. doi: 10.1016/j.jbior.2016.09.007. Epub 2016 Sep 29. Adv Biol Regul. 2017. PMID: 27720306 Free PMC article. Review.
Cited by
-
Sphingosine kinase 1-associated autophagy differs between neurons and astrocytes.Cell Death Dis. 2018 May 1;9(5):521. doi: 10.1038/s41419-018-0599-5. Cell Death Dis. 2018. PMID: 29743513 Free PMC article.
-
Shear stress-exposed pulmonary artery endothelial cells fail to upregulate HSP70 in chronic thromboembolic pulmonary hypertension.PLoS One. 2020 Dec 3;15(12):e0242960. doi: 10.1371/journal.pone.0242960. eCollection 2020. PLoS One. 2020. PMID: 33270690 Free PMC article.
-
Sphingolipids in Ventilator Induced Lung Injury: Role of Sphingosine-1-Phosphate Lyase.Int J Mol Sci. 2018 Jan 1;19(1):114. doi: 10.3390/ijms19010114. Int J Mol Sci. 2018. PMID: 29301259 Free PMC article.
-
IQGAP1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia.Am J Physiol Lung Cell Mol Physiol. 2012 Jul 1;303(1):L12-9. doi: 10.1152/ajplung.00375.2011. Epub 2012 May 4. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22561460 Free PMC article.
-
Sphingolipids in lung endothelial biology and regulation of vascular integrity.Handb Exp Pharmacol. 2013;(216):201-26. doi: 10.1007/978-3-7091-1511-4_10. Handb Exp Pharmacol. 2013. PMID: 23563658 Free PMC article. Review.
References
-
- Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–768. - PubMed
-
- Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003;31:1216–1219. - PubMed
-
- Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

