Peripheral delivery of a CNS targeted, metalo-protease reduces aβ toxicity in a mouse model of Alzheimer's disease
- PMID: 21304989
- PMCID: PMC3031588
- DOI: 10.1371/journal.pone.0016575
Peripheral delivery of a CNS targeted, metalo-protease reduces aβ toxicity in a mouse model of Alzheimer's disease
Erratum in
-
Correction: Peripheral Delivery of a CNS Targeted, Metalo-Protease Reduces Aβ Toxicity in a Mouse Model of Alzheimer's Disease.PLoS One. 2025 Aug 20;20(8):e0330647. doi: 10.1371/journal.pone.0330647. eCollection 2025. PLoS One. 2025. PMID: 40833947 Free PMC article.
Abstract
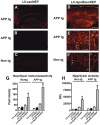
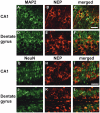
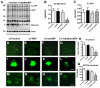
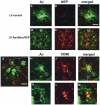
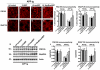
Alzheimer's disease (AD), an incurable, progressive neurodegenerative disorder, is the most common form of dementia. Therapeutic options have been elusive due to the inability to deliver proteins across the blood-brain barrier (BBB). In order to improve the therapeutic potential for AD, we utilized a promising new approach for delivery of proteins across the BBB. We generated a lentivirus vector expressing the amyloid β-degrading enzyme, neprilysin, fused to the ApoB transport domain and delivered this by intra-peritoneal injection to amyloid protein precursor (APP) transgenic model of AD. Treated mice had reduced levels of Aβ, reduced plaques and increased synaptic density in the CNS. Furthermore, mice treated with the neprilysin targeting the CNS had a reversal of memory deficits. Thus, the addition of the ApoB transport domain to the secreted neprilysin generated a non-invasive therapeutic approach that may be a potential treatment in patients with AD.
Conflict of interest statement
Figures


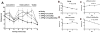
Similar articles
-
A Novel Design of a Portable Birdcage via Meander Line Antenna (MLA) to Lower Beta Amyloid (Aβ) in Alzheimer's Disease.IEEE J Transl Eng Health Med. 2025 Apr 10;13:158-173. doi: 10.1109/JTEHM.2025.3559693. eCollection 2025. IEEE J Transl Eng Health Med. 2025. PMID: 40657533 Free PMC article.
-
Somatostatin therapy, neprilysin activation, and amyloid beta reduction: A novel approach for Alzheimer's treatment.Biomed Pharmacother. 2025 Aug;189:118325. doi: 10.1016/j.biopha.2025.118325. Epub 2025 Jul 8. Biomed Pharmacother. 2025. PMID: 40633205
-
Acute targeting of N-terminal tau protein has long-lasting beneficial effects in Tg2576 APP/Aβ mouse model by reducing cognitive impairment, cerebral Aβ-amyloidosis, synaptic remodeling and microgliosis later in life.Acta Neuropathol Commun. 2025 May 29;13(1):121. doi: 10.1186/s40478-025-02022-y. Acta Neuropathol Commun. 2025. PMID: 40442822 Free PMC article.
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Active Targeted Nanoemulsions for Repurposing of Tegaserod in Alzheimer's Disease Treatment.Pharmaceutics. 2021 Oct 6;13(10):1626. doi: 10.3390/pharmaceutics13101626. Pharmaceutics. 2021. PMID: 34683919 Free PMC article.
-
Blood-brain barrier penetrating neprilysin degrades monomeric amyloid-beta in a mouse model of Alzheimer's disease.Alzheimers Res Ther. 2022 Dec 5;14(1):180. doi: 10.1186/s13195-022-01132-2. Alzheimers Res Ther. 2022. PMID: 36471433 Free PMC article.
-
Targeting Ligands Deliver Model Drug Cargo into the Central Nervous System along Autonomic Neurons.ACS Nano. 2019 Oct 22;13(10):10961-10971. doi: 10.1021/acsnano.9b01515. Epub 2019 Oct 7. ACS Nano. 2019. PMID: 31589023 Free PMC article.
-
Systemic Central Nervous System (CNS)-targeted Delivery of Neuropeptide Y (NPY) Reduces Neurodegeneration and Increases Neural Precursor Cell Proliferation in a Mouse Model of Alzheimer Disease.J Biol Chem. 2016 Jan 22;291(4):1905-1920. doi: 10.1074/jbc.M115.678185. Epub 2015 Nov 30. J Biol Chem. 2016. PMID: 26620558 Free PMC article.
-
Is Entresto good for the brain?World J Cardiol. 2017 Jul 26;9(7):594-599. doi: 10.4330/wjc.v9.i7.594. World J Cardiol. 2017. PMID: 28824789 Free PMC article. Review.
References
-
- Ashford JW. APOE genotype effects on Alzheimer's disease onset and epidemiology. J Mol Neurosci. 2004;23:157–165. - PubMed
-
- Terry R, Hansen L, Masliah E. Structural basis of the cognitive alterations in Alzheimer disease. In: Terry R, Katzman R, editors. Alzheimer disease. New York: Raven Press; 1994. pp. 179–196.
-
- Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. - PubMed
-
- Howell S, Nalbantoglu J, Crine P. Neutral endopeptidase can hydrolyze beta-amyloid(1-40) but shows no effect on beta-amyloid precursor protein metabolism. Peptides. 1995;16:647–652. - PubMed
-
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, et al. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

