Propofol directly increases tau phosphorylation
- PMID: 21304998
- PMCID: PMC3031597
- DOI: 10.1371/journal.pone.0016648
Propofol directly increases tau phosphorylation
Abstract
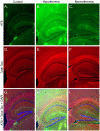
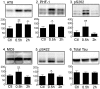
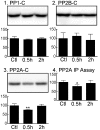
In Alzheimer's disease (AD) and other tauopathies, the microtubule-associated protein tau can undergo aberrant hyperphosphorylation potentially leading to the development of neurofibrillary pathology. Anesthetics have been previously shown to induce tau hyperphosphorylation through a mechanism involving hypothermia-induced inhibition of protein phosphatase 2A (PP2A) activity. However, the effects of propofol, a common clinically used intravenous anesthetic, on tau phosphorylation under normothermic conditions are unknown. We investigated the effects of a general anesthetic dose of propofol on levels of phosphorylated tau in the mouse hippocampus and cortex under normothermic conditions. Thirty min following the administration of propofol 250 mg/kg i.p., significant increases in tau phosphorylation were observed at the AT8, CP13, and PHF-1 phosphoepitopes in the hippocampus, as well as at AT8, PHF-1, MC6, pS262, and pS422 epitopes in the cortex. However, we did not detect somatodendritic relocalization of tau. In both brain regions, tau hyperphosphorylation persisted at the AT8 epitope 2 h following propofol, although the sedative effects of the drug were no longer evident at this time point. By 6 h following propofol, levels of phosphorylated tau at AT8 returned to control levels. An initial decrease in the activity and expression of PP2A were observed, suggesting that PP2A inhibition is at least partly responsible for the hyperphosphorylation of tau at multiple sites following 30 min of propofol exposure. We also examined tau phosphorylation in SH-SY5Y cells transfected to overexpress human tau. A 1 h exposure to a clinically relevant concentration of propofol in vitro was also associated with tau hyperphosphorylation. These findings suggest that propofol increases tau phosphorylation both in vivo and in vitro under normothermic conditions, and further studies are warranted to determine the impact of this anesthetic on the acceleration of neurofibrillary pathology.
Conflict of interest statement
Figures




References
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. - PubMed
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. - PubMed
-
- Montejo de Garcini E, Serrano L, Avila J. Self assembly of microtubule associated protein tau into filaments resembling those found in Alzheimer disease. Biochem Biophys Res Commun. 1986;141:790–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

