Immunisation with recombinant PfEMP1 domains elicits functional rosette-inhibiting and phagocytosis-inducing antibodies to Plasmodium falciparum
- PMID: 21305024
- PMCID: PMC3031562
- DOI: 10.1371/journal.pone.0016414
Immunisation with recombinant PfEMP1 domains elicits functional rosette-inhibiting and phagocytosis-inducing antibodies to Plasmodium falciparum
Abstract
Background: Rosetting is a Plasmodium falciparum virulence factor implicated in the pathogenesis of life-threatening malaria. Rosetting occurs when parasite-derived P. falciparum Erythrocyte Membrane Protein One (PfEMP1) on the surface of infected erythrocytes binds to human receptors on uninfected erythrocytes. PfEMP1 is a possible target for a vaccine to induce antibodies to inhibit rosetting and prevent severe malaria.
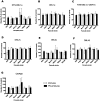
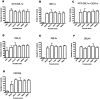
Methodology/findings: We examined the vaccine potential of the six extracellular domains of a rosette-mediating PfEMP1 variant (ITvar9/R29var1 from the R29 parasite strain) by immunizing rabbits with recombinant proteins expressed in E. coli. Antibodies raised to each domain were tested for surface fluorescence with live infected erythrocytes, rosette inhibition and phagocytosis-induction. Antibodies to all PfEMP1 domains recognized the surface of live infected erythrocytes down to low concentrations (0.02-1.56 µg/ml of total IgG). Antibodies to all PfEMP1 domains except for the second Duffy-Binding-Like region inhibited rosetting (50% inhibitory concentration 0.04-4 µg/ml) and were able to opsonize and induce phagocytosis of infected erythrocytes at low concentrations (1.56-6.25 µg/ml). Antibodies to the N-terminal region (NTS-DBL1α) were the most effective in all assays. All antibodies were specific for the R29 parasite strain, and showed no functional activity against five other rosetting strains.
Conclusions/significance: These results are encouraging for vaccine development as they show that potent antibodies can be generated to recombinant PfEMP1 domains that will inhibit rosetting and induce phagocytosis of infected erythrocytes. However, further work is needed on rosetting mechanisms and cross-reactivity in field isolates to define a set of PfEMP1 variants that could induce functional antibodies against a broad range of P. falciparum rosetting parasites.
Conflict of interest statement
Figures
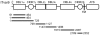




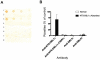
Similar articles
-
Phagocytosis-inducing antibodies to Plasmodium falciparum upon immunization with a recombinant PfEMP1 NTS-DBL1α domain.Malar J. 2016 Aug 17;15(1):416. doi: 10.1186/s12936-016-1459-3. Malar J. 2016. PMID: 27531359 Free PMC article.
-
Immunogenicity of the Plasmodium falciparum PfEMP1-VarO Adhesin: Induction of Surface-Reactive and Rosette-Disrupting Antibodies to VarO Infected Erythrocytes.PLoS One. 2015 Jul 29;10(7):e0134292. doi: 10.1371/journal.pone.0134292. eCollection 2015. PLoS One. 2015. PMID: 26222304 Free PMC article.
-
Immunization with PfEMP1-DBL1alpha generates antibodies that disrupt rosettes and protect against the sequestration of Plasmodium falciparum-infected erythrocytes.Vaccine. 2004 Jul 29;22(21-22):2701-12. doi: 10.1016/j.vaccine.2004.02.015. Vaccine. 2004. PMID: 15246600
-
Three Is a Crowd - New Insights into Rosetting in Plasmodium falciparum.Trends Parasitol. 2017 Apr;33(4):309-320. doi: 10.1016/j.pt.2016.12.012. Epub 2017 Jan 18. Trends Parasitol. 2017. PMID: 28109696 Review.
-
Molecular mechanisms and biological importance of Plasmodium falciparum erythrocyte rosetting.Mem Inst Oswaldo Cruz. 1992;87 Suppl 3:323-9. doi: 10.1590/s0074-02761992000700054. Mem Inst Oswaldo Cruz. 1992. PMID: 1285315 Review.
Cited by
-
Functional analysis of monoclonal antibodies against the Plasmodium falciparum PfEMP1-VarO adhesin.Malar J. 2016 Jan 15;15:28. doi: 10.1186/s12936-015-1016-5. Malar J. 2016. PMID: 26772184 Free PMC article.
-
Progress and prospects for blood-stage malaria vaccines.Expert Rev Vaccines. 2016 Jun;15(6):765-81. doi: 10.1586/14760584.2016.1141680. Epub 2016 Feb 3. Expert Rev Vaccines. 2016. PMID: 26760062 Free PMC article. Review.
-
A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells.Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):E1772-81. doi: 10.1073/pnas.1120461109. Epub 2012 May 22. Proc Natl Acad Sci U S A. 2012. PMID: 22619330 Free PMC article.
-
Malaria-induced bacteremia as a consequence of multiple parasite survival strategies.Curr Res Microb Sci. 2021 May 8;2:100036. doi: 10.1016/j.crmicr.2021.100036. eCollection 2021 Dec. Curr Res Microb Sci. 2021. PMID: 34841327 Free PMC article. Review.
-
Non-O ABO blood group genotypes differ in their associations with Plasmodium falciparum rosetting and severe malaria.PLoS Genet. 2023 Sep 14;19(9):e1010910. doi: 10.1371/journal.pgen.1010910. eCollection 2023 Sep. PLoS Genet. 2023. PMID: 37708213 Free PMC article.
References
-
- Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, et al. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. - PubMed
-
- Treutiger CJ, Hedlund I, Helmby H, Carlson J, Jepson A, et al. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992;46:503–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

