Effects of flavonoids on glycosaminoglycan synthesis: implications for substrate reduction therapy in Sanfilippo disease and other mucopolysaccharidoses
- PMID: 21305347
- PMCID: PMC3070076
- DOI: 10.1007/s11011-011-9233-2
Effects of flavonoids on glycosaminoglycan synthesis: implications for substrate reduction therapy in Sanfilippo disease and other mucopolysaccharidoses
Abstract
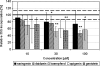
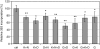
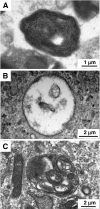
Sanfilippo disease (mucopolysaccharidosis type III, MPS III) is a severe metabolic disorder caused by accumulation of heparan sulfate (HS), one of glycosaminoglycans (GAGs), due to a genetic defect resulting in a deficiency of GAG hydrolysis. This disorder is characterized as the most severe neurological form of MPS, revealing rapid deterioration of brain functions. Among therapeutic approaches for MPS III, one of the most promising appears to be the substrate reduction therapy (SRT). Genistein (5, 7-dihydroxy-3- (4-hydroxyphenyl)-4H-1-benzopyran-4-one) is an isoflavone that has been used in SRT for MPS III. In this report, we tested effects of other flavonoids (apigenin, daidzein, kaempferol and naringenin) on GAG synthesis. Their cytotoxicity and anti-proliferation features were also tested. We found that daidzein and kaempferol inhibited GAG synthesis significantly. Moreover, these compounds were able to reduce lysosomal storage in MPS IIIA fibroblasts. Interestingly, although genistein is believed to inhibit GAG synthesis by blocking the tyrosine kinase activity of the epidermal growth factor receptor, we found that effects of other flavonoids were not due to this mechanism. In fact, combinations of various flavonoids resulted in significantly more effective inhibition of GAG synthesis than the use of any of these compounds alone. These results, together with results published recently by others, suggest that combination of flavonoids can be considered as a method for improvement of efficiency of SRT for MPS III.
Figures
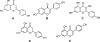
References
-
- Beck M. Mucopolysaccharidoses: clinical features and management. In: vom Dahl S, Wendel U, Strohmeyer G, editors. Genetic metabolic disorders: management, costs and sociomedical aspects. Cologne: Deutscher Arzte-Verlag; 2007. pp. 13–18.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

