A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-κB for degradation
- PMID: 21306441
- PMCID: PMC3178796
- DOI: 10.1111/j.1365-2958.2011.07568.x
A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-κB for degradation
Abstract
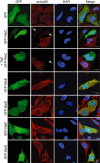
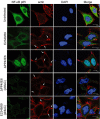
Many bacterial pathogens utilize a type III secretion system (T3SS) to inject virulence effector proteins into host cells during infection. Previously, we found that enteropathogenic Escherichia coli (EPEC) uses the type III effector, NleE, to block the inflammatory response by inhibiting IκB degradation and nuclear translocation of the p65 subunit of NF-κB. Here we screened further effectors with unknown function for their capacity to prevent p65 nuclear translocation. We observed that ectopic expression of GFP-NleC in HeLa cells led to the degradation of p65. Delivery of NleC by the T3SS of EPEC also induced degradation of p65 in infected cells as well as other NF-κB components, c-Rel and p50. Recombinant His(6) -NleC induced p65 and p50 cleavage in HeLa cell lysates and mutation of a consensus zinc metalloprotease motif, HEIIH, abrogated NleC proteolytic activity. NleC inhibited IL-8 production during prolonged EPEC infection of HeLa cells in a protease activity-dependent manner. A double nleE/nleC mutant was further impaired for its ability to inhibit IL-8 secretion than either a single nleE or a single nleC mutant. We conclude that NleC is a type III effector protease that degrades NF-κB thereby contributing the arsenal of bacterial effectors that inhibit innate immune activation.
© 2011 Blackwell Publishing Ltd.
Figures



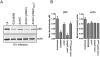
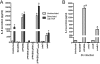
References
-
- Arbibe L, Kim DW, Batsche E, Pedron T, Mateescu B, Muchardt C, et al. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat Immunol. 2007;8:47–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

