The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults
- PMID: 21306569
- PMCID: PMC3151550
- DOI: 10.1111/j.1750-2659.2010.00183.x
The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults
Abstract
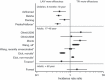
In the United States, two types of vaccines are recommended for the prevention of influenza: an intranasal live attenuated influenza vaccine (LAIV) for eligible individuals aged 2-49 years and unadjuvanted injectable trivalent inactivated vaccines (TIV) for eligible individuals aged ≥ 6 months. Several recent studies have compared the efficacy of the 2 vaccines in children and adults. In children 6 months to 18 years of age, each of the four comparative studies of LAIV and TIV demonstrated that LAIV was more protective. In individuals 17-49 years of age, most comparative studies have demonstrated that LAIV and TIV were similarly efficacious or that TIV was more efficacious. However, LAIV was shown to be more protective than TIV in new military recruits of all ages, and placebo-controlled studies in adults in 1997-1998 suggested that LAIV was more protective against the mismatched A/H3N2 strain. The relative efficacy of LAIV and TIV among young adults may vary depending on the specific population and the antigenic match between the vaccines and circulating strains. In adults 60 years of age and older, limited data suggest that the two vaccines are similarly effective. In children and adults, studies also suggest that the relative efficacy of LAIV versus TIV may increase when measured against more severe illness. Additional research comparing LAIV and TIV is needed in adults and would also be valuable in older children and adolescents. Studies should examine the role of pre-existing immunity as well as vaccine impact on influenza illness of varying severity.
© 2010 Blackwell Publishing Ltd.
Figures
References
-
- Fiore AE, Shay DK, Broder K et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1–52. - PubMed
-
- Ashkenazi S, Vertruyen A, Aristegui J et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879. - PubMed
-
- Fleming DM, Crovari P, Wahn U et al. Comparison of the efficacy and safety of live attenuated cold‐adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J 2006; 25:860–869. - PubMed
-
- Belshe RB, Edwards KM, Vesikari T et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

