How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines?
- PMID: 21306581
- PMCID: PMC3111672
- DOI: 10.1111/j.1476-5381.2011.01255.x
How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines?
Abstract
Given that cardiovascular safety liabilities remain a major cause of drug attrition during preclinical and clinical development, adverse drug reactions, and post-approval withdrawal of medicines, the Medical Research Council Centre for Drug Safety Science hosted a workshop to discuss current challenges in determining, understanding and addressing 'Cardiovascular Toxicity of Medicines'. This article summarizes the key discussions from the workshop that aimed to address three major questions: (i) what are the key cardiovascular safety liabilities in drug discovery, drug development and clinical practice? (ii) how good are preclinical and clinical strategies for detecting cardiovascular liabilities? and (iii) do we have a mechanistic understanding of these liabilities? It was concluded that in order to understand, address and ultimately reduce cardiovascular safety liabilities of new therapeutic agents there is an urgent need to: • Fully characterize the incidence, prevalence and impact of drug-induced cardiovascular issues at all stages of the drug development process. • Ascertain the predictive value of existing non-clinical models and assays towards the clinical outcome. • Understand the mechanistic basis of cardiovascular liabilities; by addressing areas where it is currently not possible to predict clinical outcome based on preclinical safety data. • Provide scientists in all disciplines with additional skills to enable them to better integrate preclinical and clinical data and to better understand the biological and clinical significance of observed changes. • Develop more appropriate, highly relevant and predictive tools and assays to identify and wherever feasible to eliminate cardiovascular safety liabilities from molecules and wherever appropriate to develop clinically relevant and reliable safety biomarkers.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
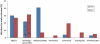

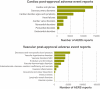
References
-
- Anon. Adverse Event Reporting System (AERS) – U.S. Food and Drug Administration – U.S. Department of Health & Human Services. 2010a. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveil... (accessed on 1st July 2010)
-
- Anon. Top Institute Pharma. 2010b. http://www.tipharma.com/about-our-institute/about-ti-pharma.html (accessed on 22nd November 2010)
-
- Anon. Cardiac Safety Research Consortium. 2010c. https://www.cardiac-safety.org/ (accessed on 22nd November 2010)
-
- Anon. Critical Path Institute's Predictive Safety Testing Consortium. 2010d. http://www.c-path.org/pstc.cfm (accessed on 22nd November 2010)
-
- Anon. Innovative Medicines Initiative. 2010e. http://www.imi.europa.eu/ (accessed on 22nd November 2010)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

