Evolution of the axial system in craniates: morphology and function of the perivertebral musculature
- PMID: 21306656
- PMCID: PMC3041741
- DOI: 10.1186/1742-9994-8-4
Evolution of the axial system in craniates: morphology and function of the perivertebral musculature
Abstract
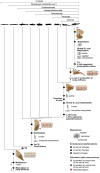
The axial musculoskeletal system represents the plesiomorphic locomotor engine of the vertebrate body, playing a central role in locomotion. In craniates, the evolution of the postcranial skeleton is characterized by two major transformations. First, the axial skeleton became increasingly functionally and morphologically regionalized. Second, the axial-based locomotion plesiomorphic for craniates became progressively appendage-based with the evolution of extremities in tetrapods. These changes, together with the transition to land, caused increased complexity in the planes in which axial movements occur and moments act on the body and were accompanied by profound changes in axial muscle function. To increase our understanding of the evolutionary transformations of the structure and function of the perivertebral musculature, this review integrates recent anatomical and physiological data (e.g., muscle fiber types, activation patterns) with gross-anatomical and kinematic findings for pivotal craniate taxa. This information is mapped onto a phylogenetic hypothesis to infer the putative character set of the last common ancestor of the respective taxa and to conjecture patterns of locomotor and muscular evolution. The increasing anatomical and functional complexity in the muscular arrangement during craniate evolution is associated with changes in fiber angulation and fiber-type distribution, i.e., increasing obliqueness in fiber orientation and segregation of fatigue-resistant fibers in deeper muscle regions. The loss of superficial fatigue-resistant fibers may be related to the profound gross anatomical reorganization of the axial musculature during the tetrapod evolution. The plesiomorphic function of the axial musculature -mobilization- is retained in all craniates. Along with the evolution of limbs and the subsequent transition to land, axial muscles additionally function to globally stabilize the trunk against inertial and extrinsic limb muscle forces as well as gravitational forces. Associated with the evolution of sagittal mobility and a parasagittal limb posture, axial muscles in mammals also stabilize the trunk against sagittal components of extrinsic limb muscle action as well as the inertia of the body's center of mass. Thus, the axial system is central to the static and dynamic control of the body posture in all craniates and, in gnathostomes, additionally provides the foundation for the mechanical work of the appendicular system.
Figures


Similar articles
-
Metabolic profile of the perivertebral muscles in small therian mammals: implications for the evolution of the mammalian trunk musculature.Zoology (Jena). 2009;112(4):279-304. doi: 10.1016/j.zool.2008.09.007. Epub 2009 Apr 16. Zoology (Jena). 2009. PMID: 19375292
-
Fiber-type composition in the perivertebral musculature of lizards: Implications for the evolution of the diapsid trunk muscles.J Morphol. 2013 Mar;274(3):294-306. doi: 10.1002/jmor.20091. Epub 2012 Oct 31. J Morphol. 2013. PMID: 23115131
-
Function of the epaxial muscles in walking, trotting and galloping dogs: implications for the evolution of epaxial muscle function in tetrapods.J Exp Biol. 2010 May;213(Pt 9):1490-502. doi: 10.1242/jeb.039487. J Exp Biol. 2010. PMID: 20400634
-
Specialize or risk disappearance - empirical evidence of anisomerism based on comparative and developmental studies of gnathostome head and limb musculature.Biol Rev Camb Philos Soc. 2015 Aug;90(3):964-78. doi: 10.1111/brv.12142. Epub 2014 Aug 30. Biol Rev Camb Philos Soc. 2015. PMID: 25174804 Review.
-
[Evolution of tetrapod locomotion].Zh Obshch Biol. 2002 Sep-Oct;63(5):426-45. Zh Obshch Biol. 2002. PMID: 12395754 Review. Russian.
Cited by
-
Ontogenetic changes of trunk muscle structure in the Japanese black salamander (Hynobius nigrescens).J Vet Med Sci. 2015 Aug;77(8):931-6. doi: 10.1292/jvms.15-0011. Epub 2015 Mar 30. J Vet Med Sci. 2015. PMID: 25816856 Free PMC article.
-
Origins of mammalian vertebral function revealed through digital bending experiments.Proc Biol Sci. 2024 Jul;291(2026):20240820. doi: 10.1098/rspb.2024.0820. Epub 2024 Jul 10. Proc Biol Sci. 2024. PMID: 38981526 Free PMC article.
-
New insights on equid locomotor evolution from the lumbar region of fossil horses.Proc Biol Sci. 2016 Apr 27;283(1829):20152947. doi: 10.1098/rspb.2015.2947. Proc Biol Sci. 2016. PMID: 27122554 Free PMC article.
-
Quantitative axial myology in two constricting snakes: Lampropeltis holbrooki and Pantherophis obsoletus.J Anat. 2018 Jun;232(6):1016-1024. doi: 10.1111/joa.12799. Epub 2018 Feb 27. J Anat. 2018. PMID: 29484639 Free PMC article.
-
The Human Neck is Part of the Musculoskeletal Core: Cervical Muscles Help Stabilize the Pelvis During Running and Jumping.Integr Org Biol. 2022 Jun 2;4(1):obac021. doi: 10.1093/iob/obac021. eCollection 2022. Integr Org Biol. 2022. PMID: 35854827 Free PMC article.
References
-
- Witmer LM. In: Functional morphology in vertebrate paleontology. Thomason, JJ, editor. Cambridge University Press, New York; 1995. The Extant Phylogenetic Bracket and the importance of reconstructing soft tissues in fossils; pp. 19–33.
-
- Hennig W. Phylogenetic systematics. University of Illinois Press, Urbana, IL, USA; 1966. pp. 1–280.
-
- Mottram SL, Comerford MJ. Stability dysfunction and low back pain. J Orthop Med. 1998;20:13–18.
-
- Schilling N. Characteristics of paravertebral muscles - fibre type distribution pattern in Ochotona rufescens (Mammalia: Lagomorpha) J Zool Syst Evol Res. 2005;43:38–48. doi: 10.1111/j.1439-0469.2004.00295.x. - DOI
LinkOut - more resources
Full Text Sources

