T cell receptor recognition of self and foreign antigens in the induction of autoimmunity
- PMID: 21306912
- PMCID: PMC3073734
- DOI: 10.1016/j.smim.2011.01.007
T cell receptor recognition of self and foreign antigens in the induction of autoimmunity
Abstract
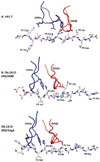
The major histocompatibility complex (MHC) on human chromosome 6 represents the most important genetic locus for a number of common human autoimmune diseases. Specific alleles that differ from closely related alleles by only one or a few amino acids in the peptide binding groove are frequently strongly associated with disease susceptibility, raising the important question of which peptide presentation events are critical in disease initiation and progression. This review will cover a number of topics pertinent to this fundamental question, including MHC linked disease susceptibility to autoimmune diseases, molecular mechanisms for the role of MHC molecules in autoimmune diseases as well as the recognition of self and microbial peptides by self-reactive T cell receptors (TCRs).
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
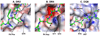
References
-
- Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. - PubMed
-
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009 - PMC - PubMed
-
- MacKay K, Eyre S, Myerscough A, Milicic A, Barton A, Laval S, Barrett J, Lee D, White S, John S, Brown MA, Bell J, Silman A, Ollier W, Wordsworth P, Worthington J. Whole-genome linkage analysis of rheumatoid arthritis susceptibility loci in 252 affected sibling pairs in the United Kingdom. Arthritis Rheum. 2002;46:632–639. - PubMed
-
- Oksenberg JR, Barcellos LF, Cree BA, Baranzini SE, Bugawan TL, Khan O, Lincoln RR, Swerdlin A, Mignot E, Lin L, Goodin D, Erlich HA, Schmidt S, Thomson G, Reich DE, Pericak-Vance MA, Haines JL, Hauser SL. Mapping multiple sclerosis susceptibility to the HLA-DR locus in African Americans. Am J Hum Genet. 2004;74:160–167. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

