A role for tRNA(His) guanylyltransferase (Thg1)-like proteins from Dictyostelium discoideum in mitochondrial 5'-tRNA editing
- PMID: 21307182
- PMCID: PMC3062173
- DOI: 10.1261/rna.2517111
A role for tRNA(His) guanylyltransferase (Thg1)-like proteins from Dictyostelium discoideum in mitochondrial 5'-tRNA editing
Abstract
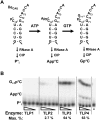
Genes with sequence similarity to the yeast tRNA(His) guanylyltransferase (Thg1) gene have been identified in all three domains of life, and Thg1 family enzymes are implicated in diverse processes, ranging from tRNA(His) maturation to 5'-end repair of tRNAs. All of these activities take advantage of the ability of Thg1 family enzymes to catalyze 3'-5' nucleotide addition reactions. Although many Thg1-containing organisms have a single Thg1-related gene, certain eukaryotic microbes possess multiple genes with sequence similarity to Thg1. Here we investigate the activities of four Thg1-like proteins (TLPs) encoded by the genome of the slime mold, Dictyostelium discoideum (a member of the eukaryotic supergroup Amoebozoa). We show that one of the four TLPs is a bona fide Thg1 ortholog, a cytoplasmic G(-1) addition enzyme likely to be responsible for tRNA(His) maturation in D. discoideum. Two other D. discoideum TLPs exhibit biochemical activities consistent with a role for these enzymes in mitochondrial 5'-tRNA editing, based on their ability to efficiently repair the 5' ends of mitochondrial tRNA editing substrates. Although 5'-tRNA editing was discovered nearly two decades ago, the identity of the protein(s) that catalyze this activity has remained elusive. This article provides the first identification of any purified protein that appears to play a role in the 5'-tRNA editing reaction. Moreover, the presence of multiple Thg1 family members in D. discoideum suggests that gene duplication and divergence during evolution has resulted in paralogous proteins that use 3'-5' nucleotide addition reactions for diverse biological functions in the same organism.
Figures

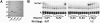
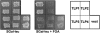



References
-
- Bullerwell CE, Gray MW 2005. In vitro characterization of a tRNA editing activity in the mitochondria of Spizellomyces punctatus, a Chytridiomycete fungus. J Biol Chem 280: 2463–2470 - PubMed
-
- Claros MG 1995. MitoProt, a Macintosh application for studying mitochondrial proteins. Comput Appl Biosci 11: 441–447 - PubMed
-
- Claros MG, Vincens P 1996. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241: 779–786 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
