A Rab11- and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA
- PMID: 21307188
- PMCID: PMC3126276
- DOI: 10.1128/JVI.02606-10
A Rab11- and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA
Abstract
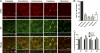
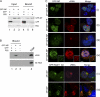
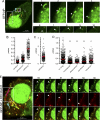
The viral RNA (vRNA) genome of influenza A virus is replicated in the nucleus, exported to the cytoplasm as ribonucleoproteins (RNPs), and trafficked to the plasma membrane through uncertain means. Using fluorescent in situ hybridization to detect vRNA as well as the live cell imaging of fluorescently labeled RNPs, we show that an early event in vRNA cytoplasmic trafficking involves accumulation near the microtubule organizing center in multiple cell types and viral strains. Here, RNPs colocalized with Rab11, a pericentriolar recycling endosome marker. Cytoplasmic RNP localization was perturbed by inhibitors of vesicular trafficking, microtubules, or the short interfering RNA-mediated depletion of Rab11. Green fluorescent protein (GFP)-tagged RNPs in living cells demonstrated rapid, bidirectional, and saltatory movement, which is characteristic of microtubule-based transport, and also cotrafficked with fluorescent Rab11. Coprecipitation experiments showed an interaction between RNPs and the GTP-bound form of Rab11, potentially mediated via the PB2 subunit of the polymerase. We propose that influenza virus RNPs are routed from the nucleus to the pericentriolar recycling endosome (RE), where they access a Rab11-dependent vesicular transport pathway to the cell periphery.
Figures








References
-
- Amorim M. J., Read E. K., Dalton R. M., Medcalf L., Digard P. 2007. Nuclear export of influenza A virus mRNAs requires ongoing RNA polymerase II activity. Traffic 8:1–11 - PubMed
-
- Arcangeletti M. C., et al. 1997. Modification of cytoskeleton and prosome networks in relation to protein synthesis in influenza A virus-infected LLC-MK2 cells. Virus Res. 51:19–34 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

