Cations but not anions regulate the responsiveness of kainate receptors
- PMID: 21307250
- PMCID: PMC6633048
- DOI: 10.1523/JNEUROSCI.4314-10.2011
Cations but not anions regulate the responsiveness of kainate receptors
Abstract
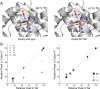
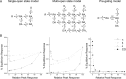
Kainate-selective ionotropic glutamate receptors are unique among ligand-gated ion channels in their obligate requirement of external anions and cations for activation. Although it is established that the degree of kainate receptor (KAR) activation is shaped by the chemical nature of the agonist molecule, the possible complementary role of external ions has yet to be examined. Here we show that external cations but not anions regulate the responsiveness to a range of full and partial agonists acting on rat GluK2 receptors. This observation is unexpected as previous work has assumed anions and cations affect KARs in an identical manner through functionally coupled binding sites. However, our data demonstrate that anion- and cation-binding pockets behave discretely. We suggest cations uniquely regulate a pregating or flipping step that impacts the closed-cleft stability of the agonist-binding domain (ABD). This model departs from a previous proposal that KAR agonist efficacy is governed by the degree of closure elicited in the ABD by ligand binding. Our findings are, however, in line with recent studies on Cys-loop ligand-gated ion channels suggesting that the "flipping" mechanism has been conserved by structurally diverse ligand-gated ion channel families as a common means of regulating neurotransmitter behavior.
Figures






References
-
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. - PubMed
-
- Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. - PubMed
-
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
