The Caenorhabditis elegans JIP3 protein UNC-16 functions as an adaptor to link kinesin-1 with cytoplasmic dynein
- PMID: 21307258
- PMCID: PMC6633058
- DOI: 10.1523/JNEUROSCI.2653-10.2011
The Caenorhabditis elegans JIP3 protein UNC-16 functions as an adaptor to link kinesin-1 with cytoplasmic dynein
Abstract
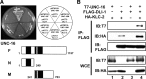
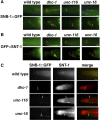
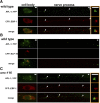
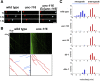
Kinesin-1 is a microtubule plus-end-directed motor that transports various cargos along the axon. Previous studies have elucidated the physical and genetic interactions between kinesin-1 and cytoplasmic dynein, a microtubule minus-end-directed motor, in neuronal cells. However, the physiological importance of kinesin-1 in the dynein-dependent retrograde transport of cargo molecules remains obscure. Here, we show that Caenorhabditis elegans kinesin-1 forms a complex with dynein via its interaction with UNC-16, which binds to the dynein light intermediate (DLI) chain. Both kinesin-1 and UNC-16 are required for localization of DLI-1 at the plus ends of nerve process microtubules. In addition, retrograde transport of APL-1 depends on kinesin-1, UNC-16, and dynein. These results suggest that kinesin-1 mediates the anterograde transport of dynein using UNC-16 as a scaffold and that dynein in turn mediates the retrograde transport of cargo molecules in vivo. Thus, UNC-16 functions as an adaptor for kinesin-1-mediated transport of dynein.
Figures




References
-
- Bowman AB, Kamal A, Ritchings BW, Philp AV, McGrail M, Gindhart JG, Goldstein LS. Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell. 2000;103:583–594. - PubMed
-
- Byrd DT, Kawasaki M, Walcoff M, Hisamoto N, Matsumoto K, Jin Y. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron. 2001;32:787–800. - PubMed
-
- Caviston JP, Holzbaur EL. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
