Polarization-sensitive descending neurons in the locust: connecting the brain to thoracic ganglia
- PMID: 21307260
- PMCID: PMC6633037
- DOI: 10.1523/JNEUROSCI.3624-10.2011
Polarization-sensitive descending neurons in the locust: connecting the brain to thoracic ganglia
Abstract
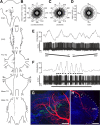
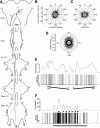
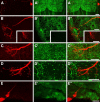
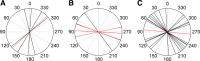
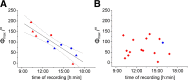
Many animal species, in particular insects, exploit the E-vector pattern of the blue sky for sun compass navigation. Like other insects, locusts detect dorsal polarized light via photoreceptors in a specialized dorsal rim area of the compound eye. Polarized light information is transmitted through several processing stages to the central complex, a brain area involved in the control of goal-directed orientation behavior. To investigate how polarized light information is transmitted to thoracic motor circuits, we studied the responses of locust descending neurons to polarized light. Three sets of polarization-sensitive descending neurons were characterized through intracellular recordings from axonal fibers in the neck connectives combined with single-cell dye injections. Two descending neurons from the brain, one with ipsilaterally and the second with contralaterally descending axon, are likely to bridge the gap between polarization-sensitive neurons in the brain and thoracic motor centers. In both neurons, E-vector tuning changed linearly with daytime, suggesting that they signal time-compensated spatial directions, an important prerequisite for navigation using celestial signals. The third type connects the suboesophageal ganglion with the prothoracic ganglion. It showed no evidence for time compensation in E-vector tuning and might play a role in flight stabilization and control of head movements.
Figures


References
-
- Bacon JP, Thompson KS, Stern M. Identified octopamine neurons provide an arousal mechanism in the locust brain. J Neurophysiol. 1995;74:2739–2743. - PubMed
-
- Brunner D, Labhart T. Behavioural evidence for polarization vision in crickets. Physiol Entomol. 1987;12:1–10.
-
- Dacke M, Nordström P, Scholtz CH. Twilight orientation to polarized light in the crepuscular dung beetle Scarabaeus zambesianus. J Exp Biol. 2003;206:1535–1543. - PubMed
-
- Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP. The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: identification by an antiserum against a synthetic PDH. Cell Tissue Res. 1987;250:377–387.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
