p75NTR regulates Abeta deposition by increasing Abeta production but inhibiting Abeta aggregation with its extracellular domain
- PMID: 21307265
- PMCID: PMC6633040
- DOI: 10.1523/JNEUROSCI.2733-10.2011
p75NTR regulates Abeta deposition by increasing Abeta production but inhibiting Abeta aggregation with its extracellular domain
Abstract
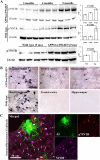
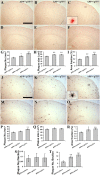
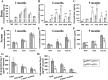
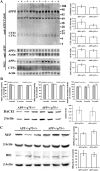
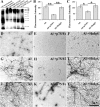
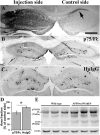
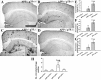
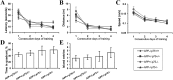
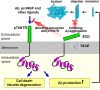
Accumulation of toxic amyloid-β (Aβ) in the cerebral cortex and hippocampus is a major pathological feature of Alzheimer's disease (AD). The neurotrophin receptor p75NTR has been proposed to mediate Aβ-induced neurotoxicity; however, its role in the development of AD remains to be clarified. The p75NTR/ExonIII-/- mice and APPSwe/PS1dE9 mice were crossed to generate transgenic AD mice with deletion of p75NTR gene. In APPSwe/PS1dE9 transgenic mice, p75NTR expression was localized in the basal forebrain neurons and degenerative neurites in neocortex, increased with aging, and further activated by Aβ accumulation. Deletion of the p75NTR gene in APPSwe/PS1dE9 mice reduced soluble Aβ levels in the brain and serum, but increased the accumulation of insoluble Aβ and Aβ plaque formation. There was no change in the levels of amyloid precursor protein (APP) and its proteolytic derivatives, or α-, β-, and γ-secretase activities, or in levels of BACE1, neprilysin (NEP), and insulin-degrading enzyme (IDE) proteins. Aβ production by cortical neurons of APPSwe/PS1dE9 mice was reduced by deletion of p75NTR gene in vitro. Recombinant extracellular domain of p75NTR attenuated the oligomerization and fibrillation of synthetic Aβ(42) peptide in vitro, and reduced local Aβ plaques after hippocampus injection in vivo. In addition, deletion of p75NTR attenuated microgliosis but increased the microhemorrhage profiles in the brain. The deletion of p75NTR did not significantly change the cognitive function of the mice up to the age of 9 months. Our data suggest that p75NTR plays a critical role in regulating Aβ levels by both increasing Aβ production and attenuating its aggregation, and they caution that a therapeutic intervention simply reducing p75NTR may exacerbate AD pathology.
Figures
Similar articles
-
Self-assembling nanofibers alter the processing of amyloid precursor protein in a transgenic mouse model of Alzheimer's disease.Neurol Res. 2015 Jan;37(1):84-91. doi: 10.1179/1743132814Y.0000000417. Epub 2014 Jul 9. Neurol Res. 2015. PMID: 25005263
-
GEPT extract reduces Abeta deposition by regulating the balance between production and degradation of Abeta in APPV717I transgenic mice.Curr Alzheimer Res. 2009 Apr;6(2):118-31. doi: 10.2174/156720509787602942. Curr Alzheimer Res. 2009. PMID: 19355846
-
Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease.J Neurosci Res. 2006 May 15;83(7):1252-61. doi: 10.1002/jnr.20818. J Neurosci Res. 2006. PMID: 16511867
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Edaravone alleviates Alzheimer's disease-type pathologies and cognitive deficits.Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):5225-30. doi: 10.1073/pnas.1422998112. Epub 2015 Apr 6. Proc Natl Acad Sci U S A. 2015. PMID: 25847999 Free PMC article.
-
Pre-Clinical Neuroprotective Evidences and Plausible Mechanisms of Sulforaphane in Alzheimer's Disease.Int J Mol Sci. 2021 Mar 13;22(6):2929. doi: 10.3390/ijms22062929. Int J Mol Sci. 2021. PMID: 33805772 Free PMC article. Review.
-
Association of rs2072446 in the NGFR gene with the risk of Alzheimer's disease and amyloid-β deposition in the brain.CNS Neurosci Ther. 2022 Dec;28(12):2218-2229. doi: 10.1111/cns.13965. Epub 2022 Sep 8. CNS Neurosci Ther. 2022. PMID: 36074475 Free PMC article.
-
Immunization Against Specific Fragments of Neurotrophin p75 Receptor Protects Forebrain Cholinergic Neurons in the Olfactory Bulbectomized Mice.J Alzheimers Dis. 2016 May 6;53(1):289-301. doi: 10.3233/JAD-160146. J Alzheimers Dis. 2016. PMID: 27163825 Free PMC article.
-
Removal of p75 Neurotrophin Receptor Expression from Cholinergic Basal Forebrain Neurons Reduces Amyloid-β Plaque Deposition and Cognitive Impairment in Aged APP/PS1 Mice.Mol Neurobiol. 2019 Jul;56(7):4639-4652. doi: 10.1007/s12035-018-1404-2. Epub 2018 Oct 29. Mol Neurobiol. 2019. PMID: 30374941
References
-
- Ahmed Z, Mazibrada G, Seabright RJ, Dent RG, Berry M, Logan A. TACE-induced cleavage of NgR and p75NTR in dorsal root ganglion cultures disinhibits outgrowth and promotes branching of neurites in the presence of inhibitory CNS myelin. FASEB J. 2006;20:1939–1941. - PubMed
-
- Barrett GL, Reid CA, Tsafoulis C, Zhu W, Williams DA, Paolini AG, Trieu J, Murphy M. Enhanced spatial memory and hippocampal long-term potentiation in p75 neurotrophin receptor knockout mice. Hippocampus. 2010;20:145–152. - PubMed
-
- Bronfman FC. Metalloproteases and gamma-secretase: new membrane partners regulating p75 neurotrophin receptor signaling? J Neurochem. 2007;103(Suppl 1):91–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
