Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer's disease
- PMID: 21307267
- PMCID: PMC3381884
- DOI: 10.1523/JNEUROSCI.4717-10.2011
Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer's disease
Abstract
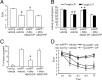
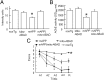
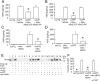
Amyloid-β (Aβ) peptide-binding alcohol dehydrogenase (ABAD), an enzyme present in neuronal mitochondria, exacerbates Aβ-induced cell stress. The interaction of ABAD with Aβ exacerbates Aβ-induced mitochondrial and neuronal dysfunction. Here, we show that inhibition of the ABAD-Aβ interaction, using a decoy peptide (DP) in vitro and in vivo, protects against aberrant mitochondrial and neuronal function and improves spatial learning/memory. Intraperitoneal administration of ABAD-DP [fused to the transduction of human immunodeficiency virus 1-transactivator (Tat) protein and linked to the mitochondrial targeting sequence (Mito) (TAT-mito-DP) to transgenic APP mice (Tg mAPP)] blocked formation of ABAD-Aβ complex in mitochondria, increased oxygen consumption and enzyme activity associated with the mitochondrial respiratory chain, attenuated mitochondrial oxidative stress, and improved spatial memory. Similar protective effects were observed in Tg mAPP mice overexpressing neuronal ABAD decoy peptide (Tg mAPP/mito-ABAD). Notably, inhibition of the ABAD-Aβ interaction significantly reduced mitochondrial Aβ accumulation. In parallel, the activity of mitochondrial Aβ-degrading enzyme PreP (presequence peptidase) was enhanced in Tg mAPP mitochondria expressing the ABAD decoy peptide. These data indicate that segregating ABAD from Aβ protects mitochondria/neurons from Aβ toxicity; thus, ABAD-Aβ interaction is an important mechanism underlying Aβ-mediated mitochondrial and neuronal perturbation. Inhibitors of ABAD-Aβ interaction may hold promise as targets for the prevention and treatment of Alzheimer's disease.
Figures


References
-
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. - PubMed
-
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. - PMC - PubMed
-
- Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36:381–386. - PubMed
-
- Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. - PubMed
-
- Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer's syndrome. Ann N Y Acad Sci. 2000;924:170–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
