Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid-mediated effect on stomatal aperture
- PMID: 21307286
- PMCID: PMC3077794
- DOI: 10.1105/tpc.110.081224
Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid-mediated effect on stomatal aperture
Abstract
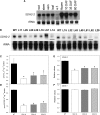
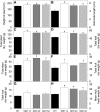
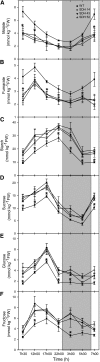
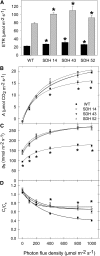
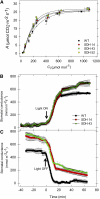
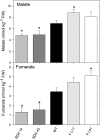
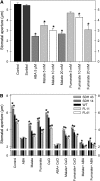
Transgenic tomato (Solanum lycopersicum) plants expressing a fragment of the Sl SDH2-2 gene encoding the iron sulfur subunit of the succinate dehydrogenase protein complex in the antisense orientation under the control of the 35S promoter exhibit an enhanced rate of photosynthesis. The rate of the tricarboxylic acid (TCA) cycle was reduced in these transformants, and there were changes in the levels of metabolites associated with the TCA cycle. Furthermore, in comparison to wild-type plants, carbon dioxide assimilation was enhanced by up to 25% in the transgenic plants under ambient conditions, and mature plants were characterized by an increased biomass. Analysis of additional photosynthetic parameters revealed that the rate of transpiration and stomatal conductance were markedly elevated in the transgenic plants. The transformants displayed a strongly enhanced assimilation rate under both ambient and suboptimal environmental conditions, as well as an elevated maximal stomatal aperture. By contrast, when the Sl SDH2-2 gene was repressed by antisense RNA in a guard cell-specific manner, changes in neither stomatal aperture nor photosynthesis were observed. The data obtained are discussed in the context of the role of TCA cycle intermediates both generally with respect to photosynthetic metabolism and specifically with respect to their role in the regulation of stomatal aperture.
Figures







References
-
- Alba R., et al. (2004). ESTs, cDNA microarrays, and gene expression profiling: Tools for dissecting plant physiology and development. Plant J. 39: 697–714 - PubMed
-
- Araújo W.L., Ishizaki K., Nunes-Nesi A., Larson T.R., Tohge T., Krahnert I., Witt S., Obata T., Schauer N., Graham I.A., Leaver C.J., Fernie A.R. (2010). Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 22: 1549–1563 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

